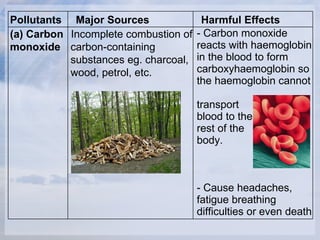
Joseph Priestley conducted experiments in the late 18th century that led to the isolation of oxygen and the development of carbonated drinks. The document then discusses the composition of air and methods for separating air into its components. It also describes common air pollutants like carbon monoxide, nitrogen oxides, and sulfur dioxide along with their major sources and harmful effects. Methods for reducing air pollution through the use of catalytic converters and flue gas desulfurization are also summarized.