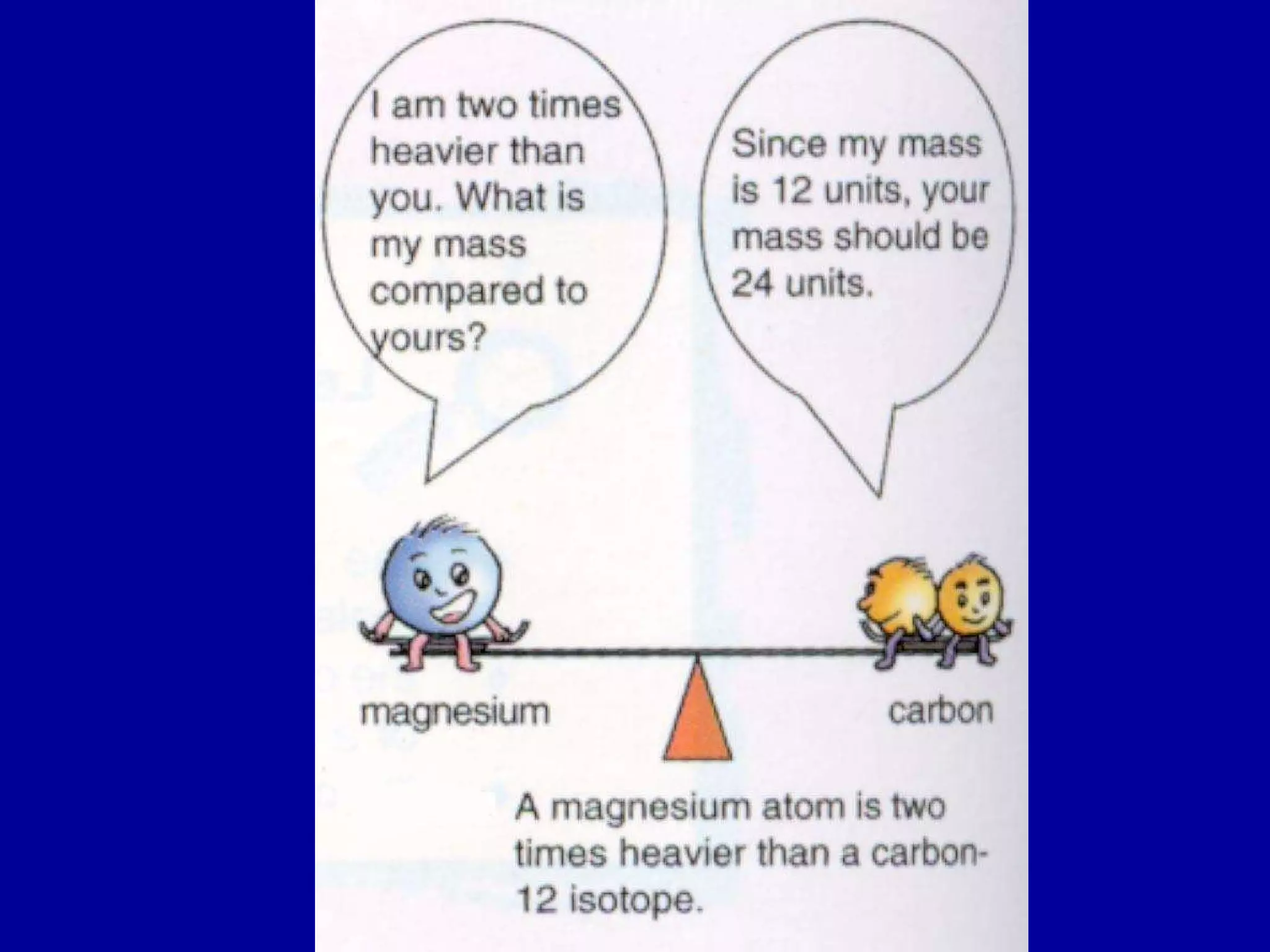
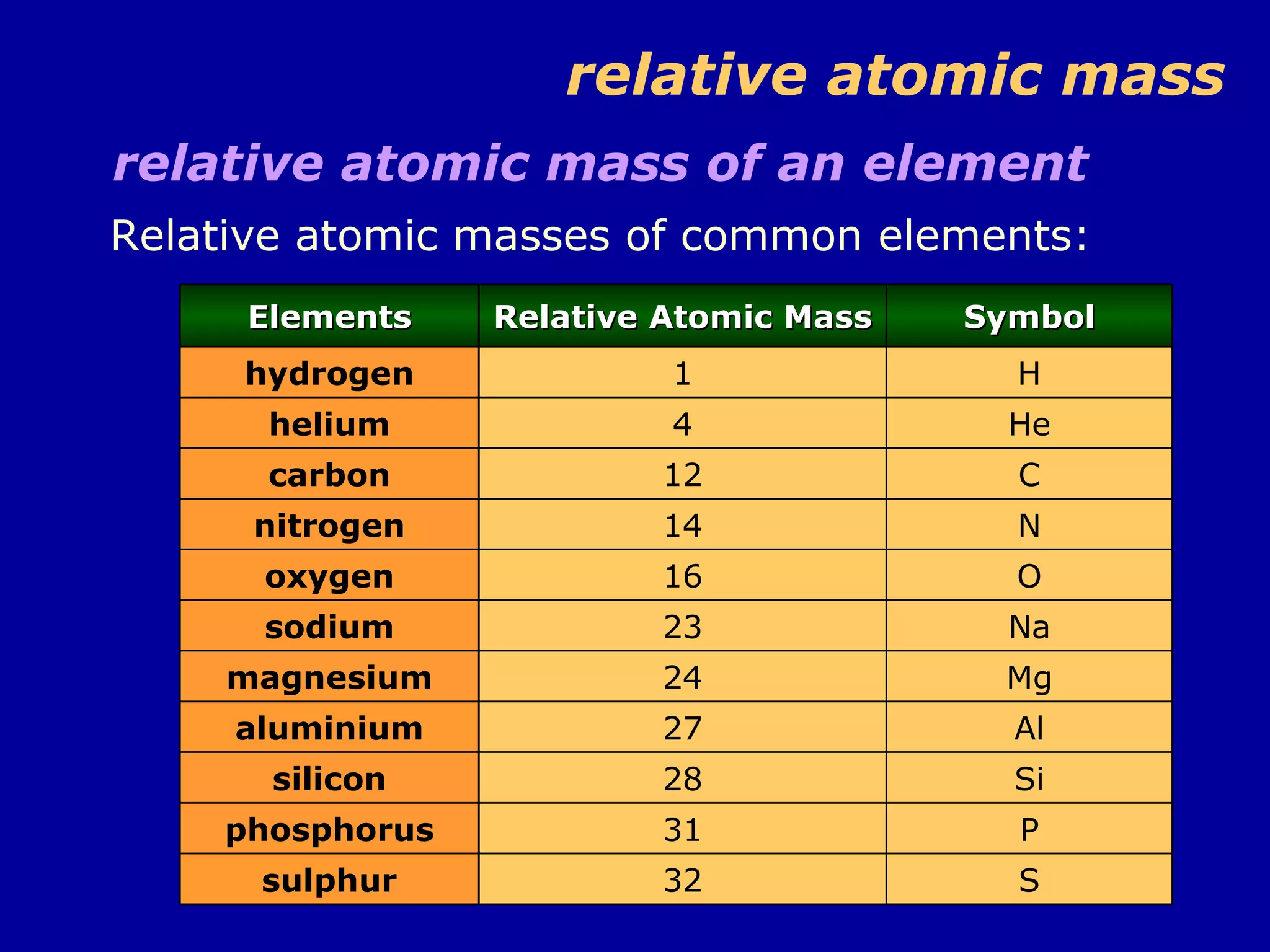
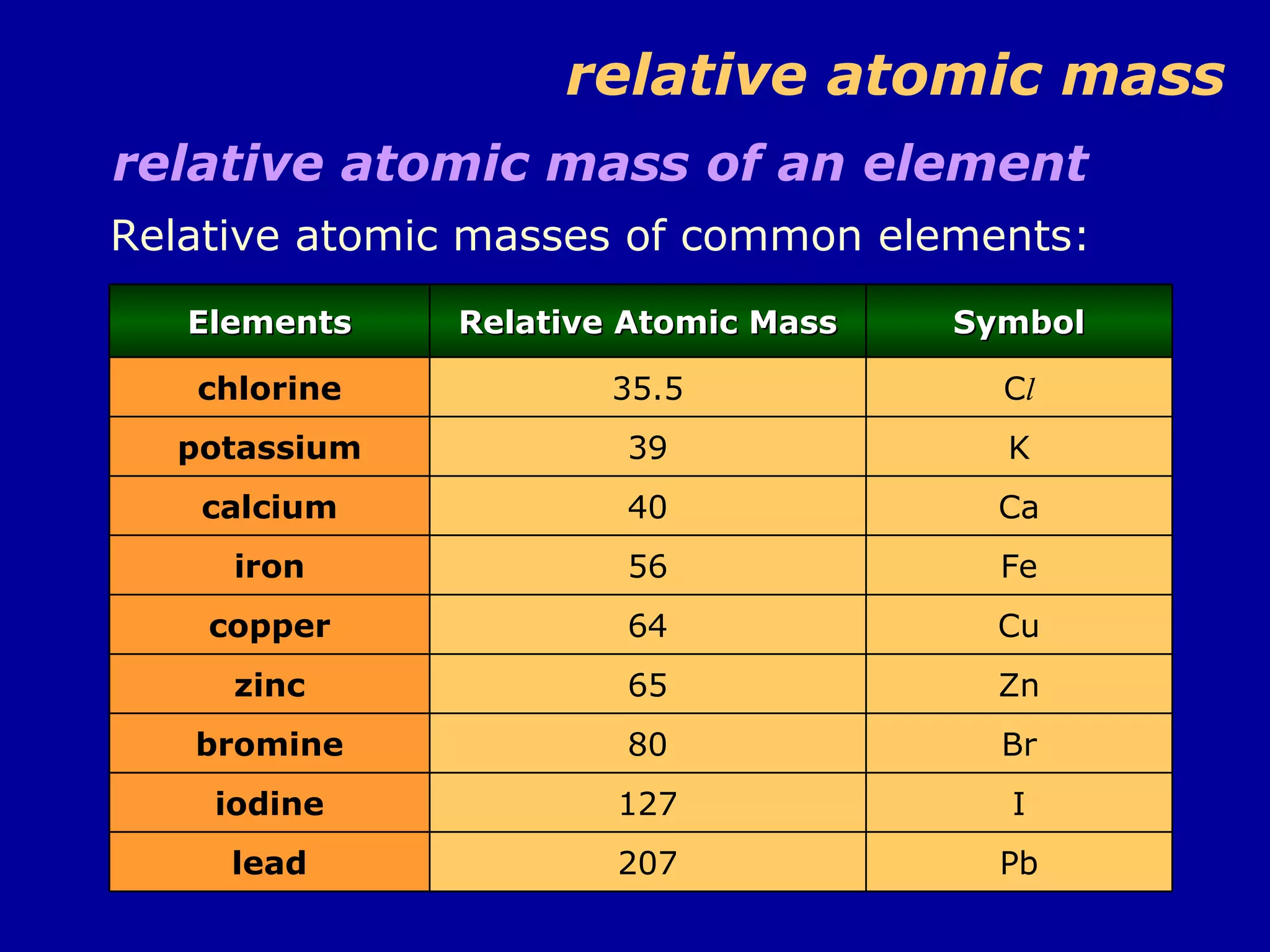
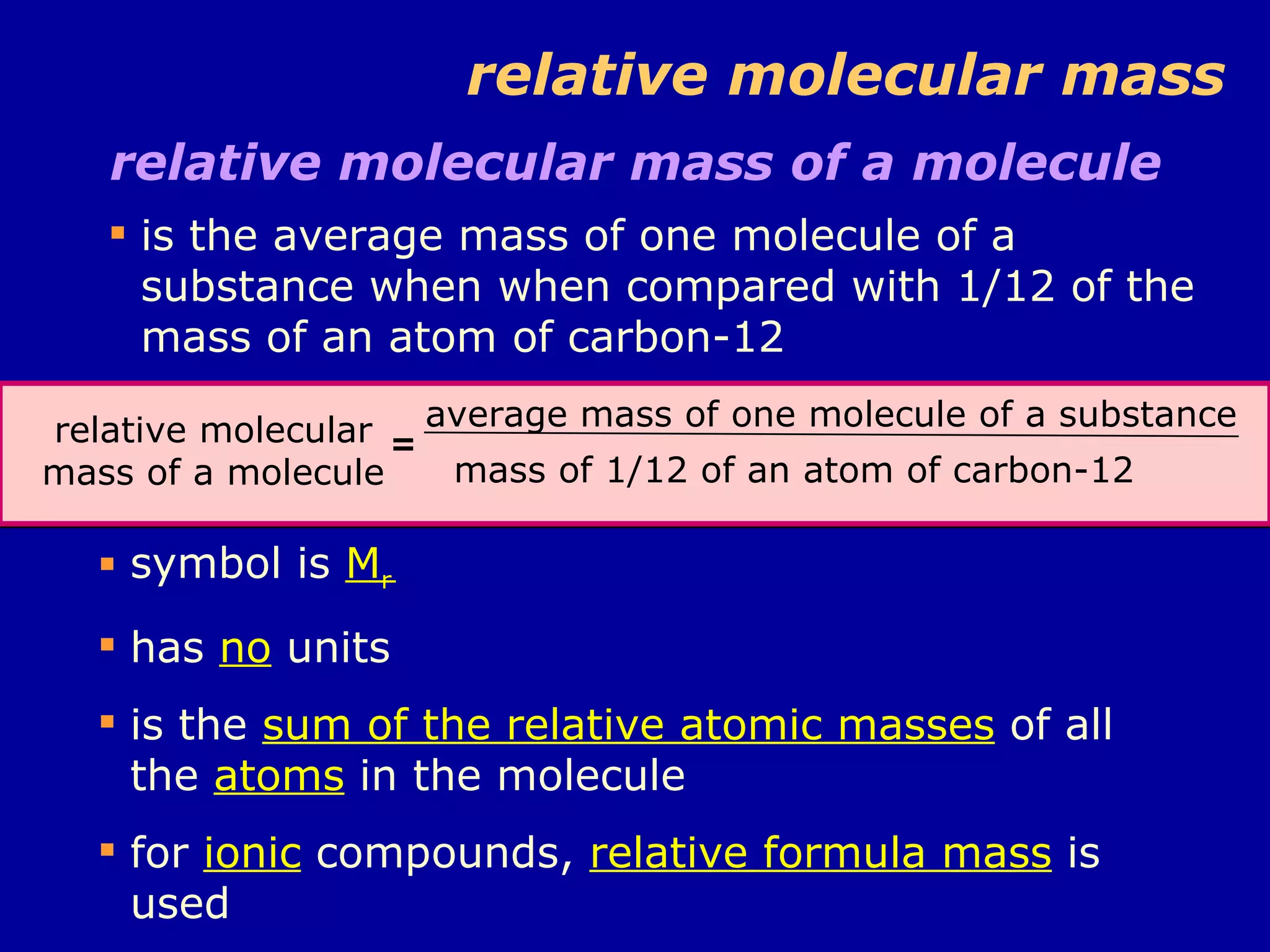
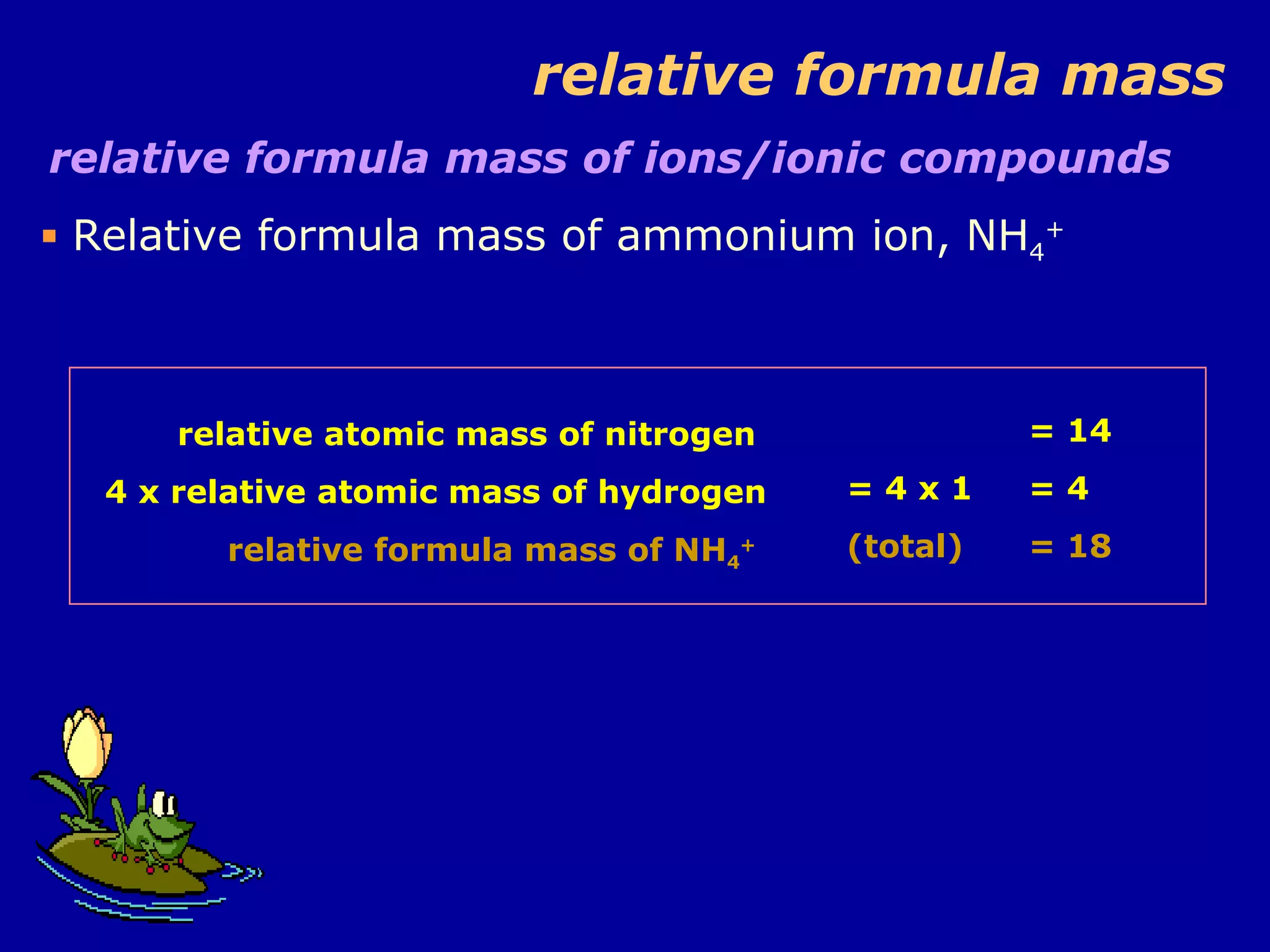
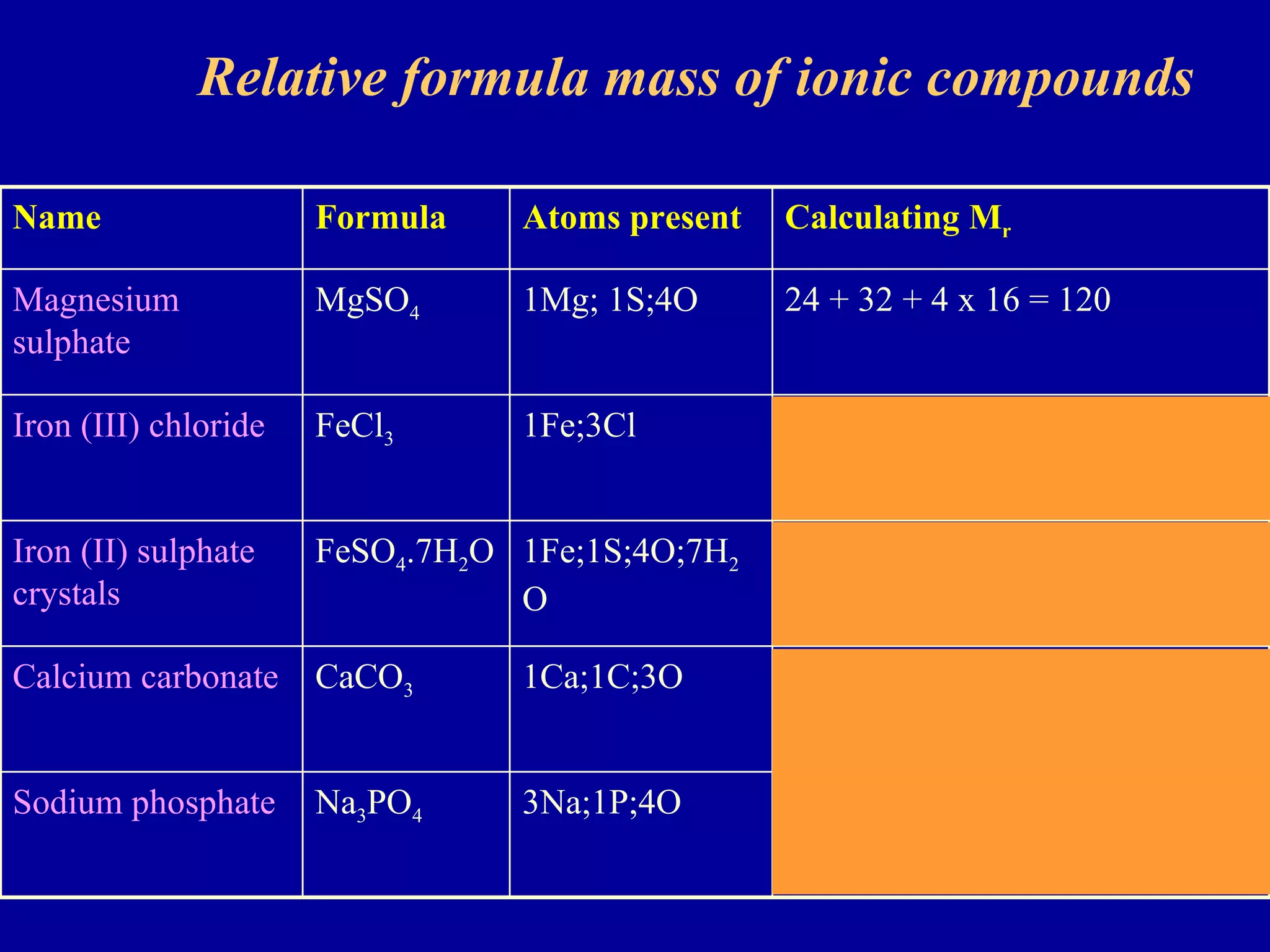
This document discusses relative atomic mass (Ar) and relative molecular mass (Mr). It defines these terms and provides examples of calculating them.
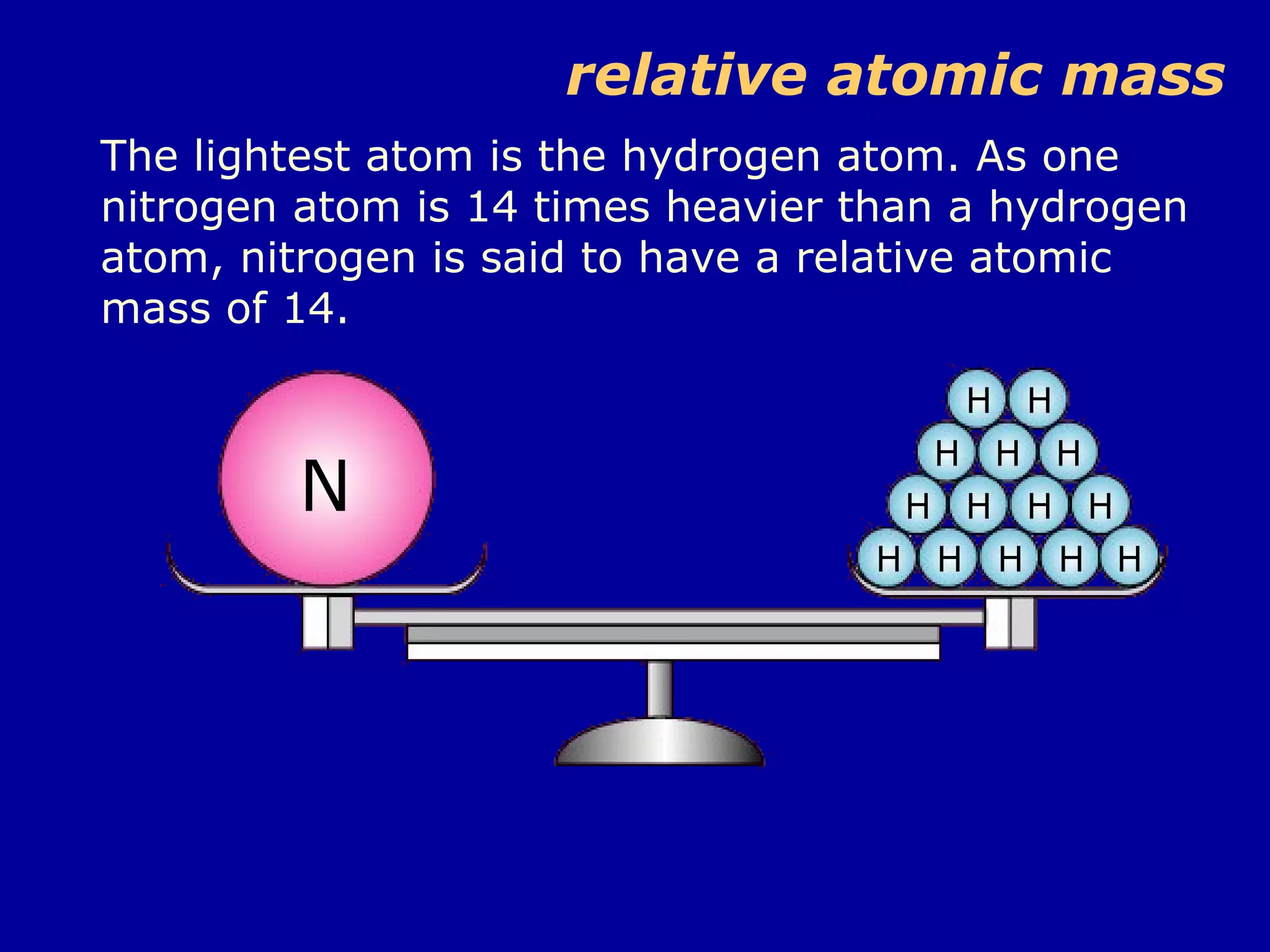
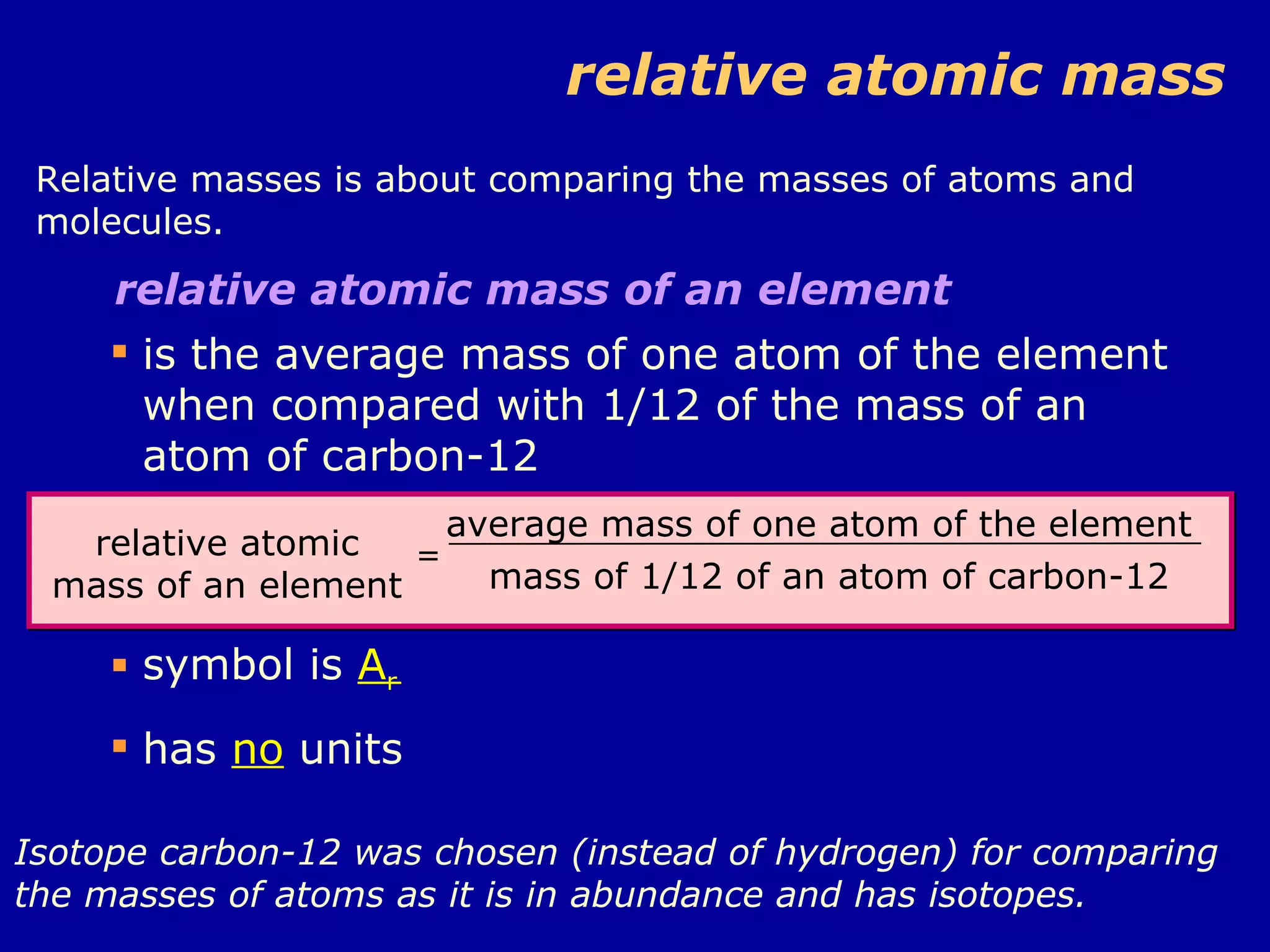
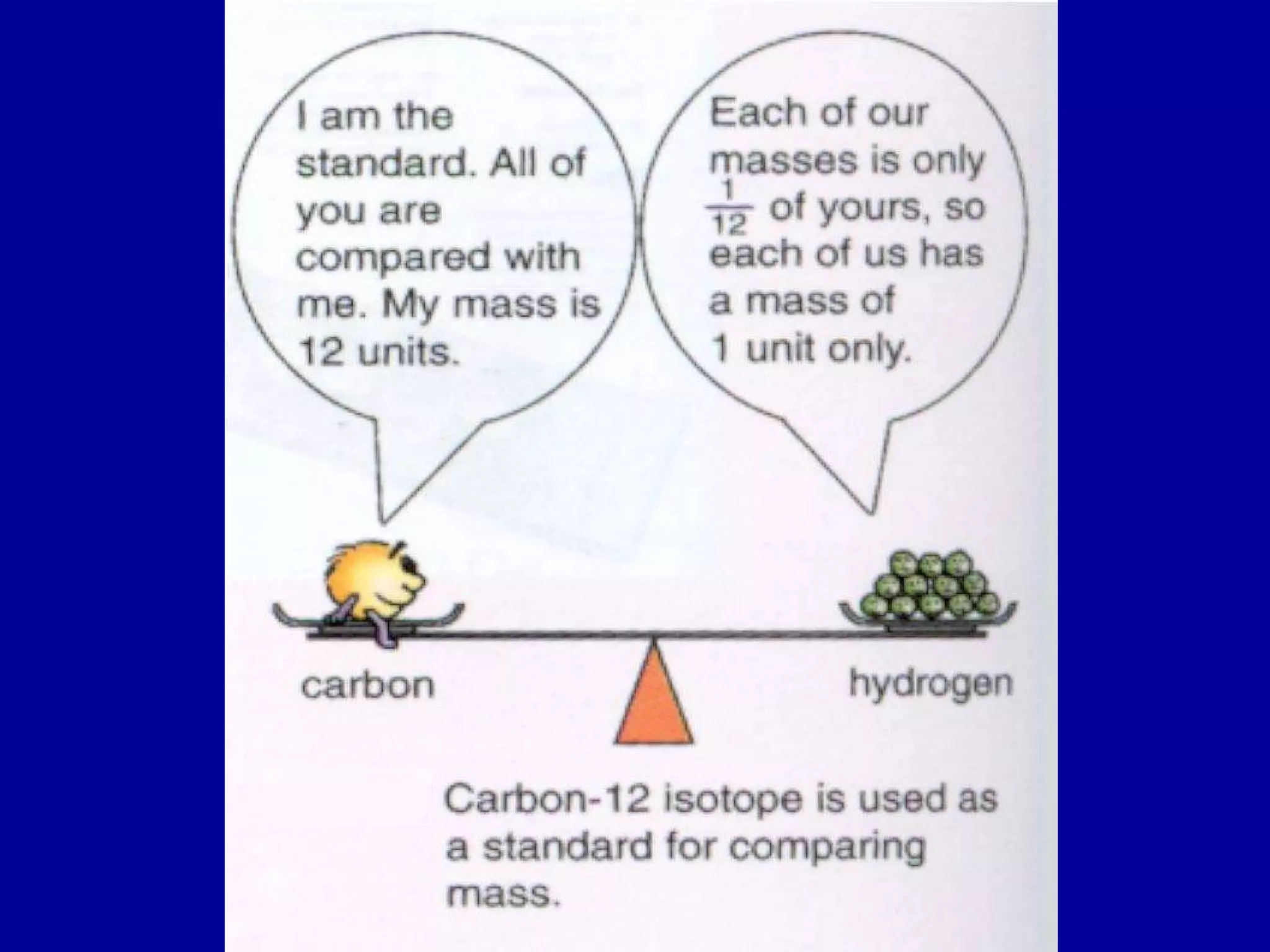
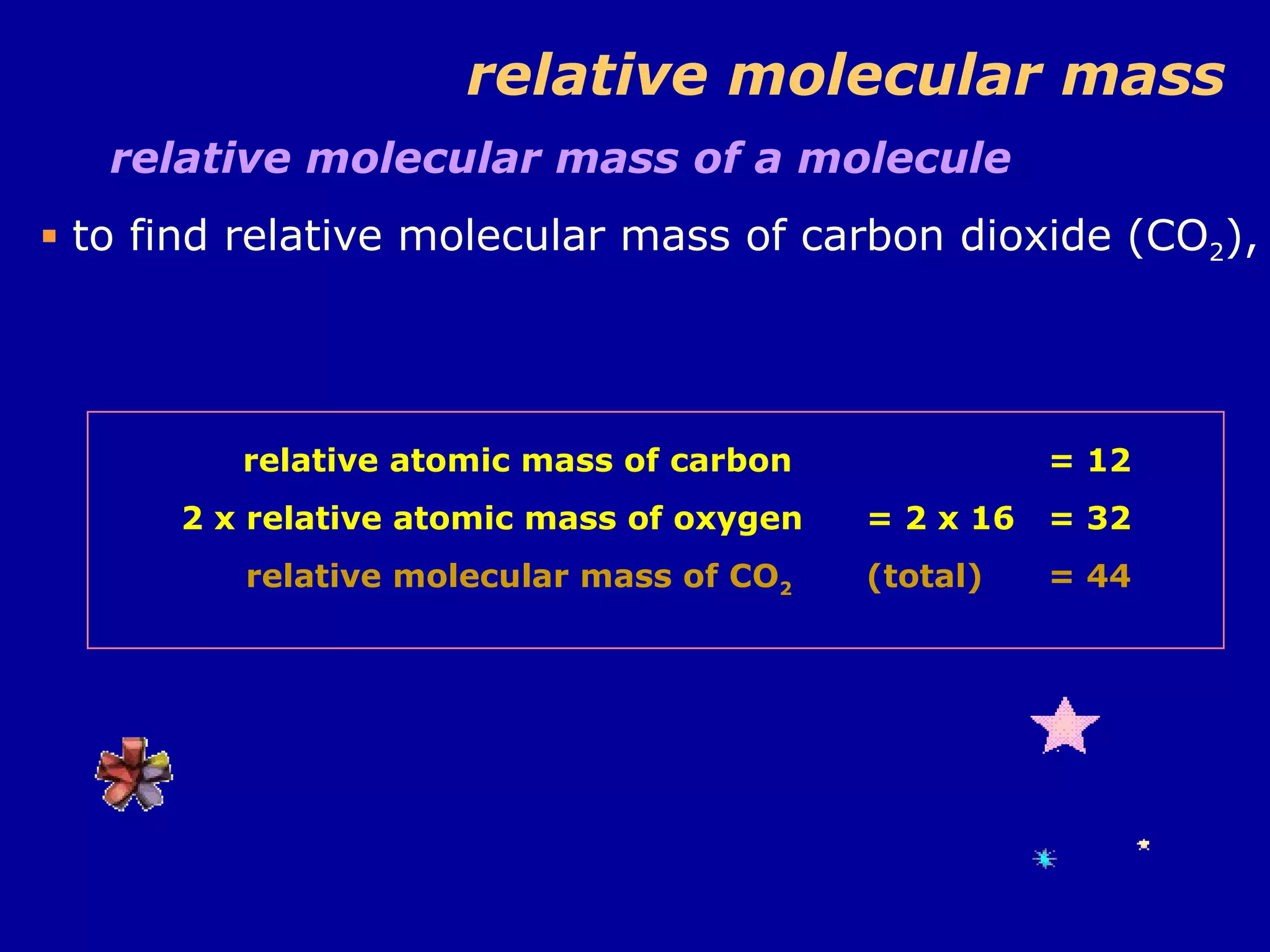
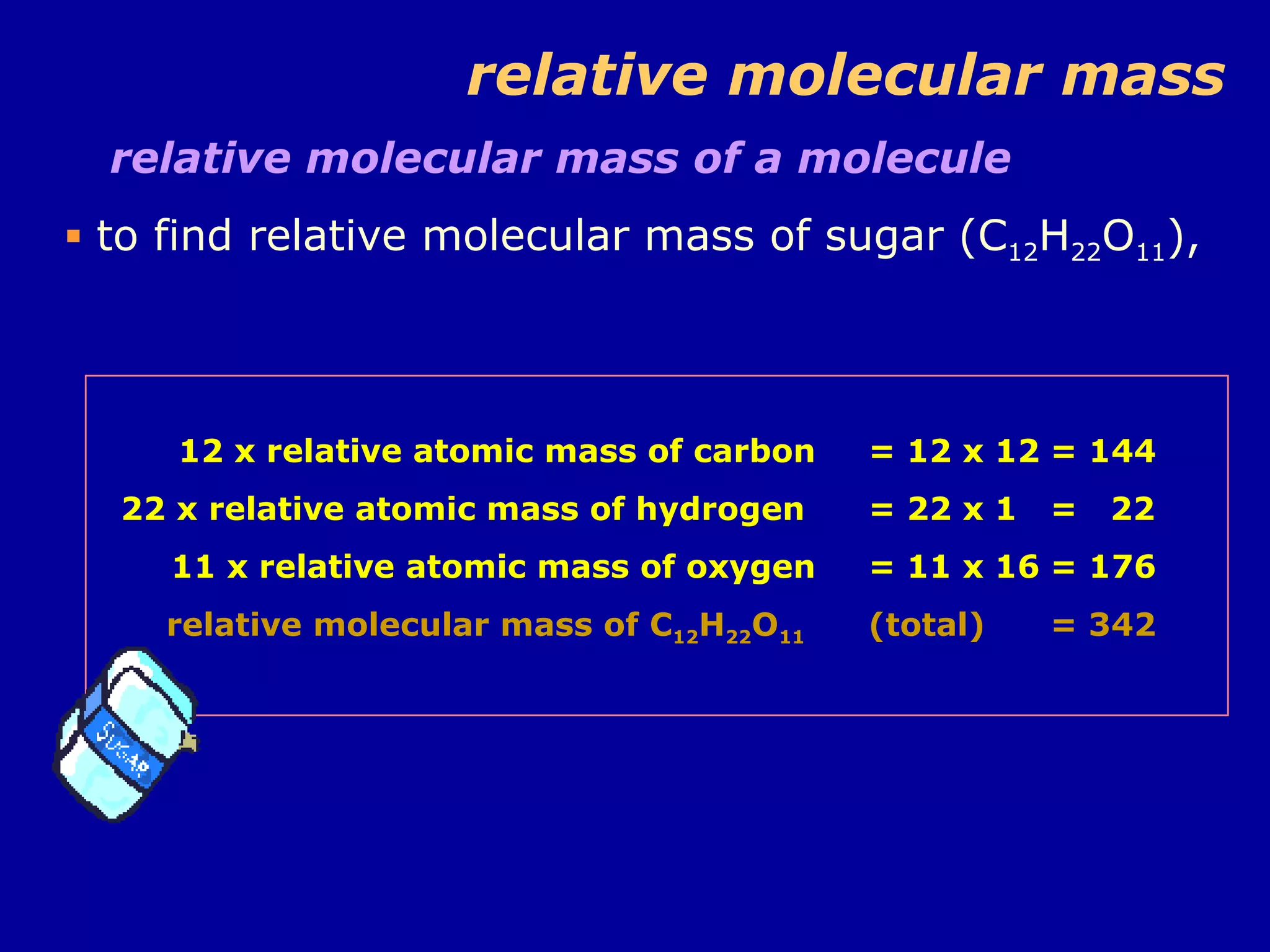
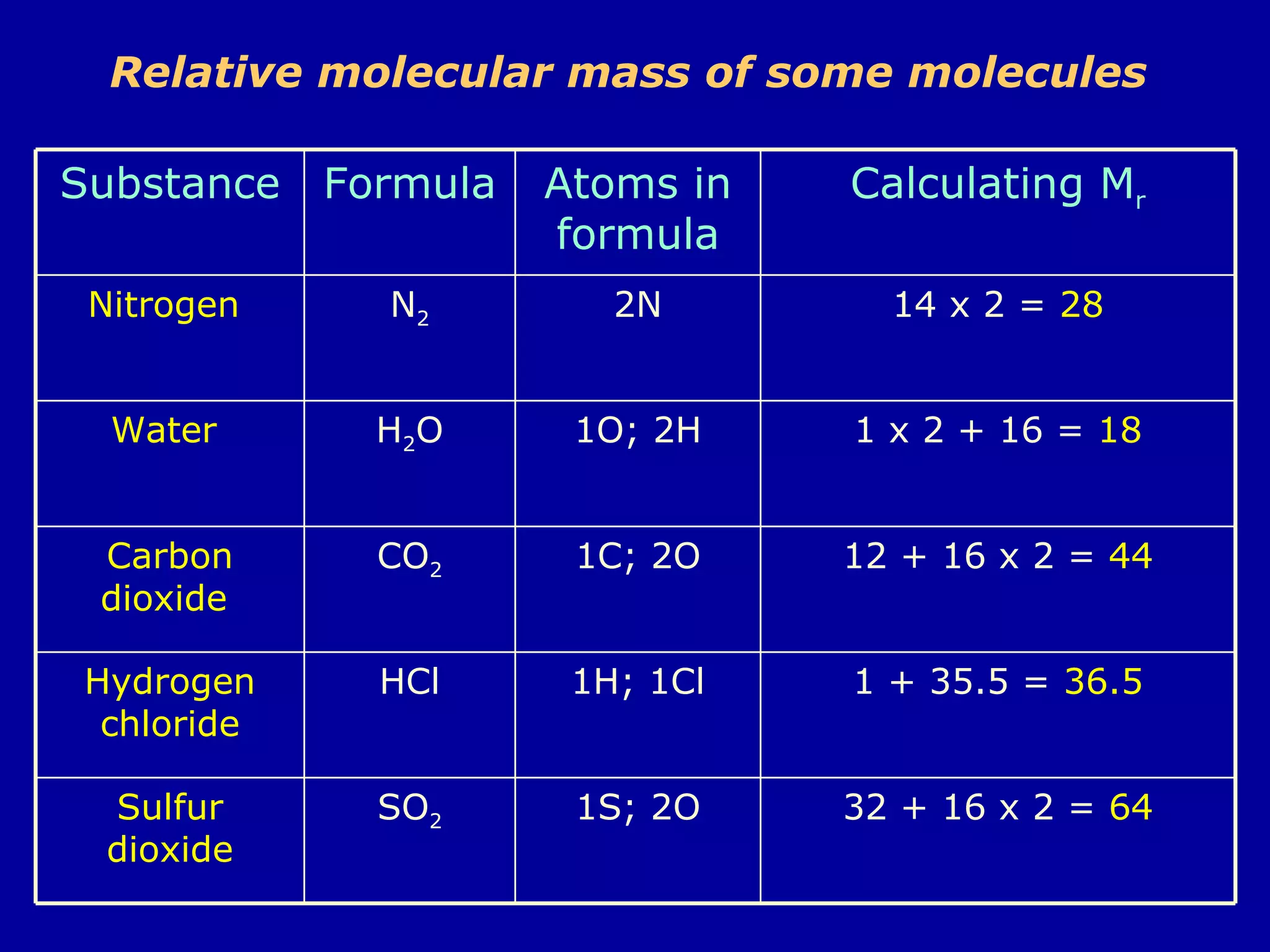
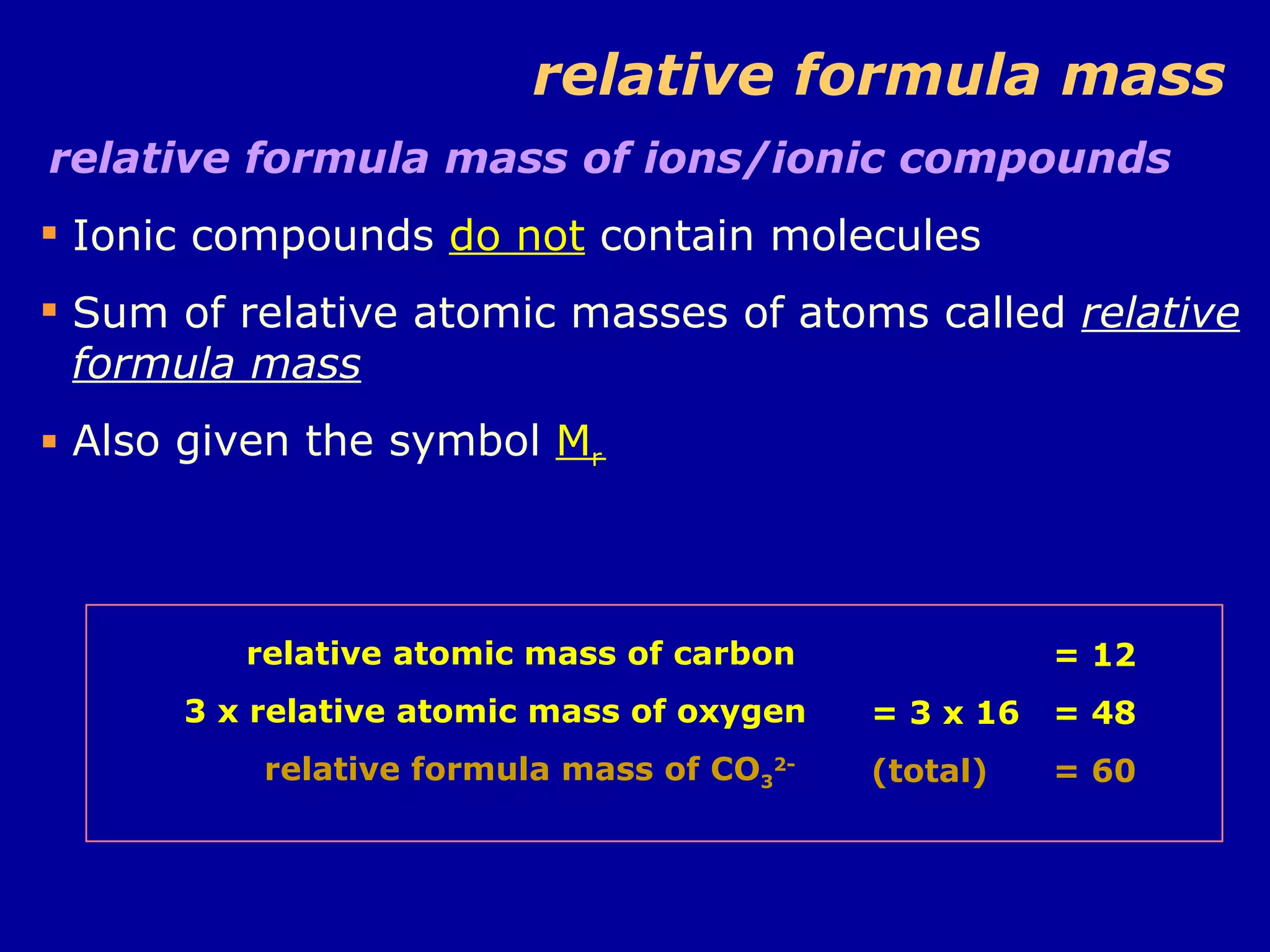
Ar is a ratio of the average mass of a given atom to 1/12 the mass of one carbon-12 atom. It allows easy comparison of atomic masses. Mr is the sum of Ar values for all atoms in a molecule or polyatomic ion. Examples are given for calculating Mr of common substances like CO2, glucose, and ionic compounds. The document also addresses why some Ar values are not whole numbers due to isotope mixtures.