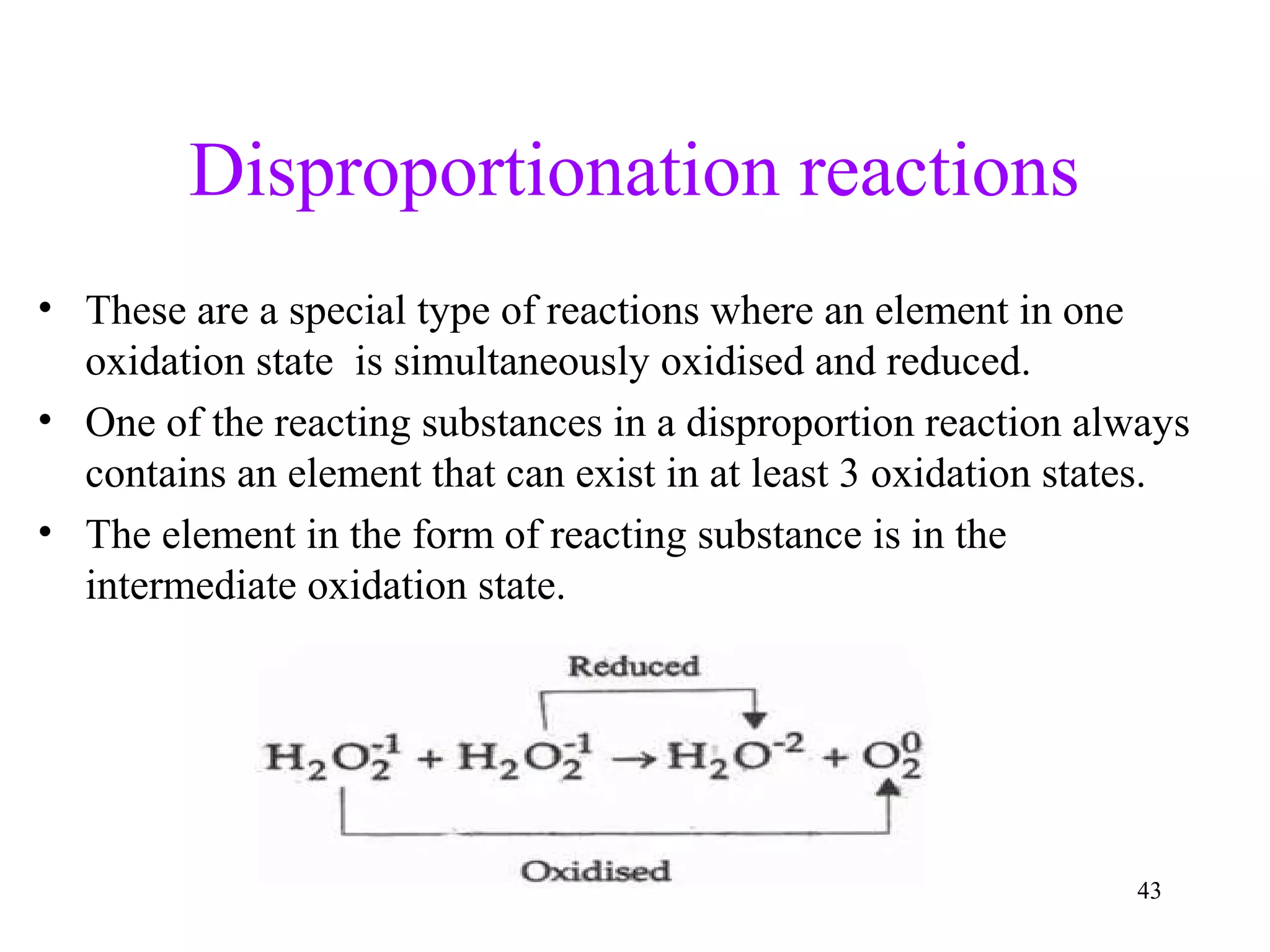
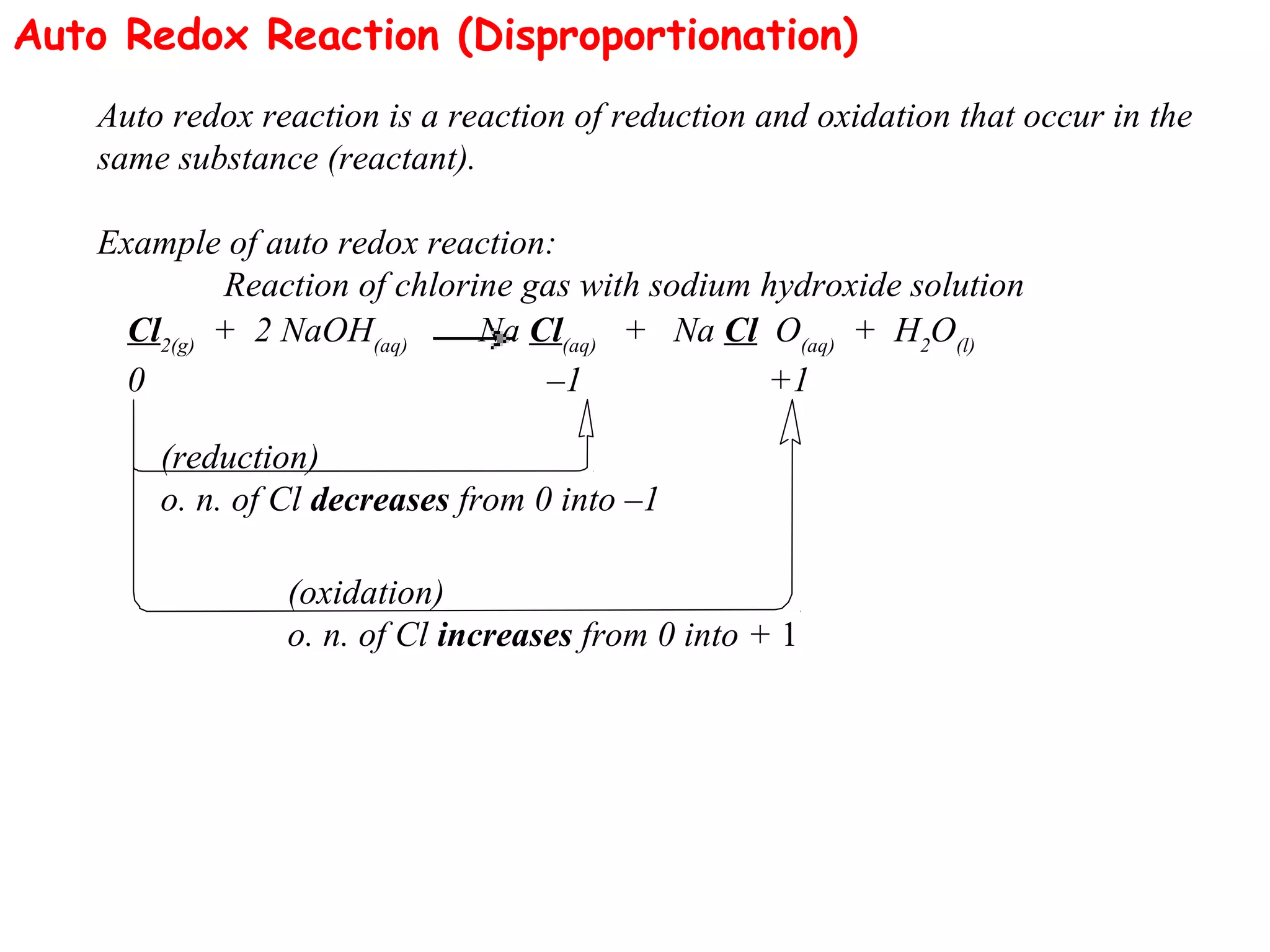
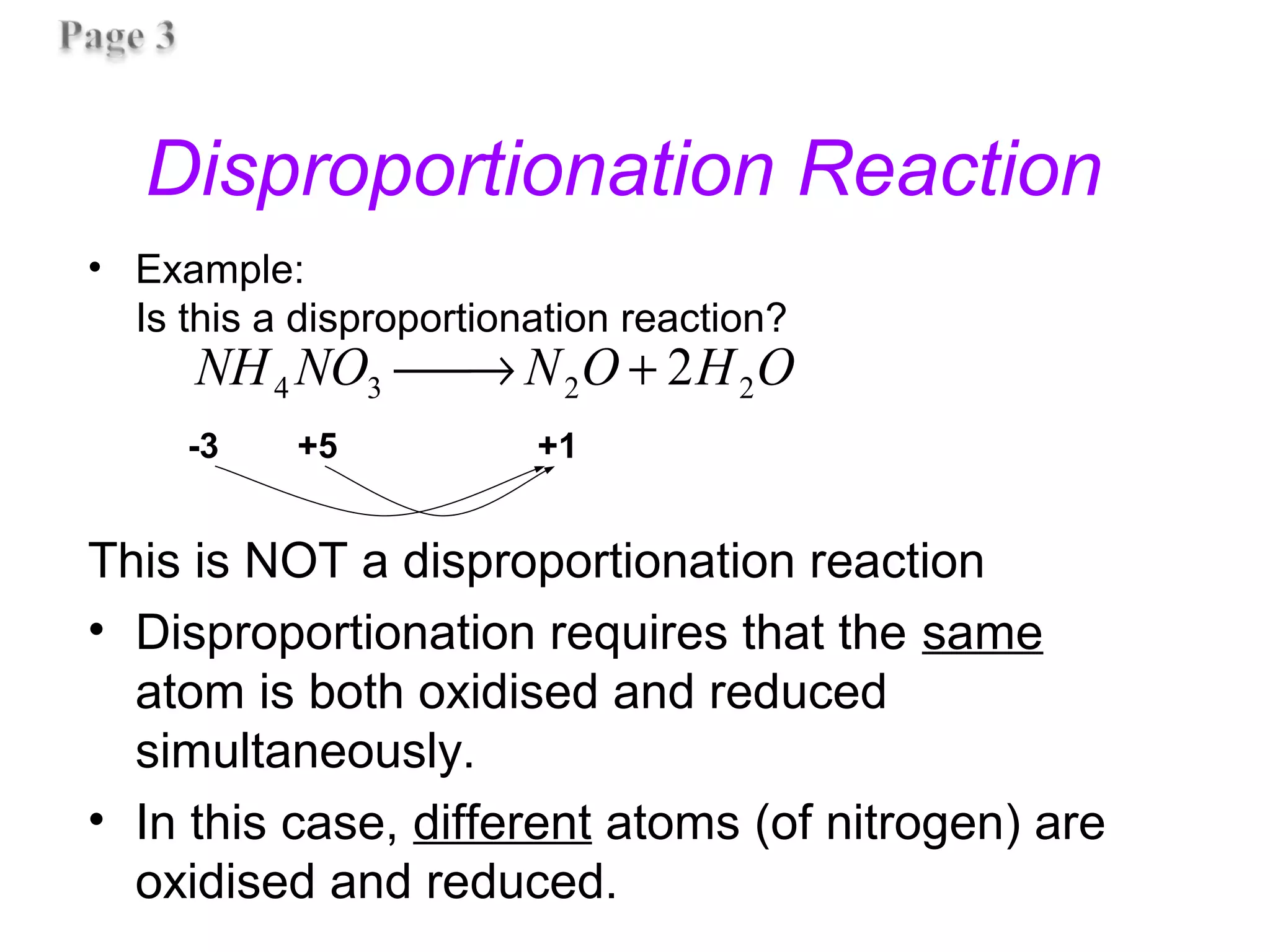
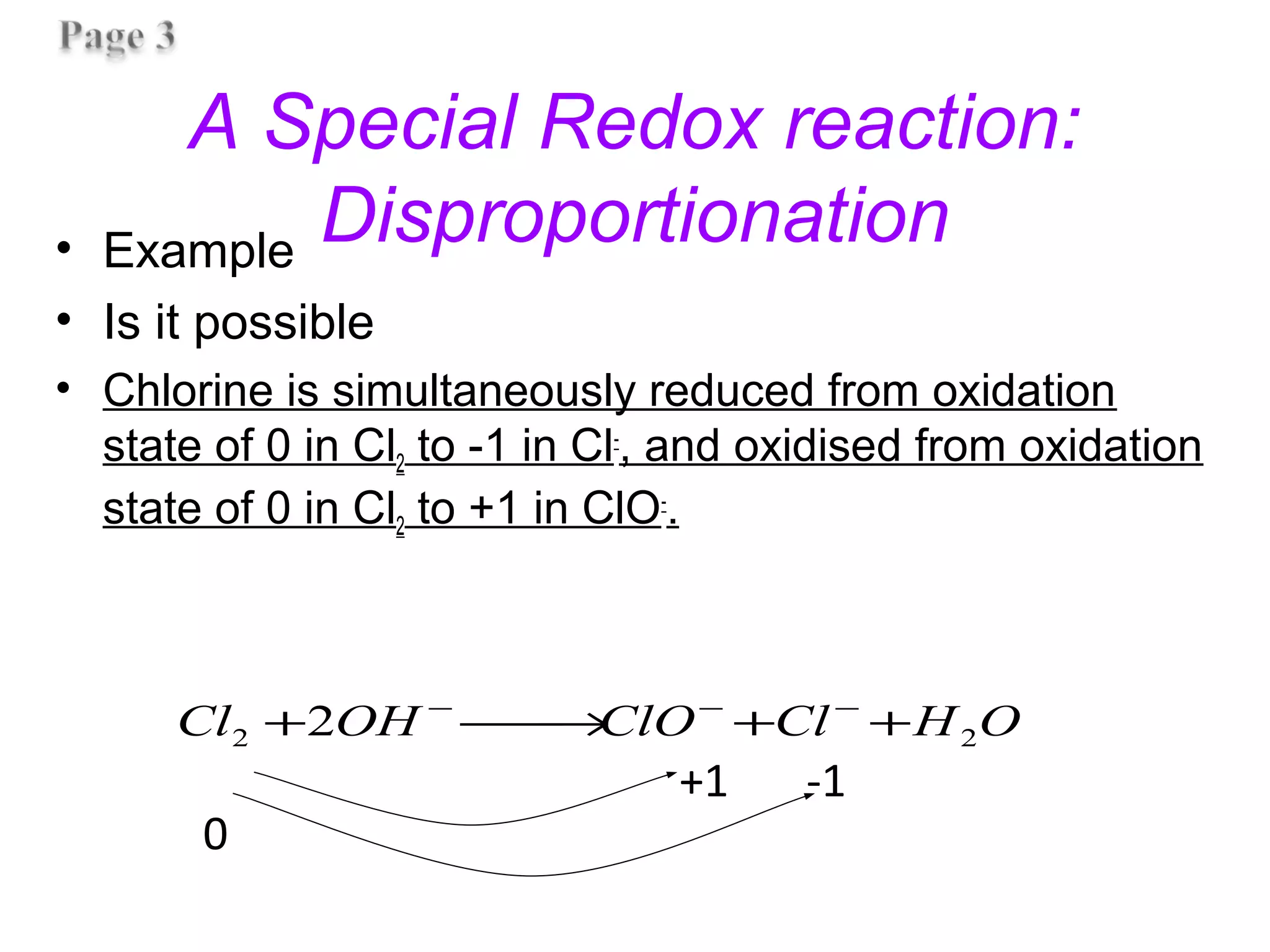
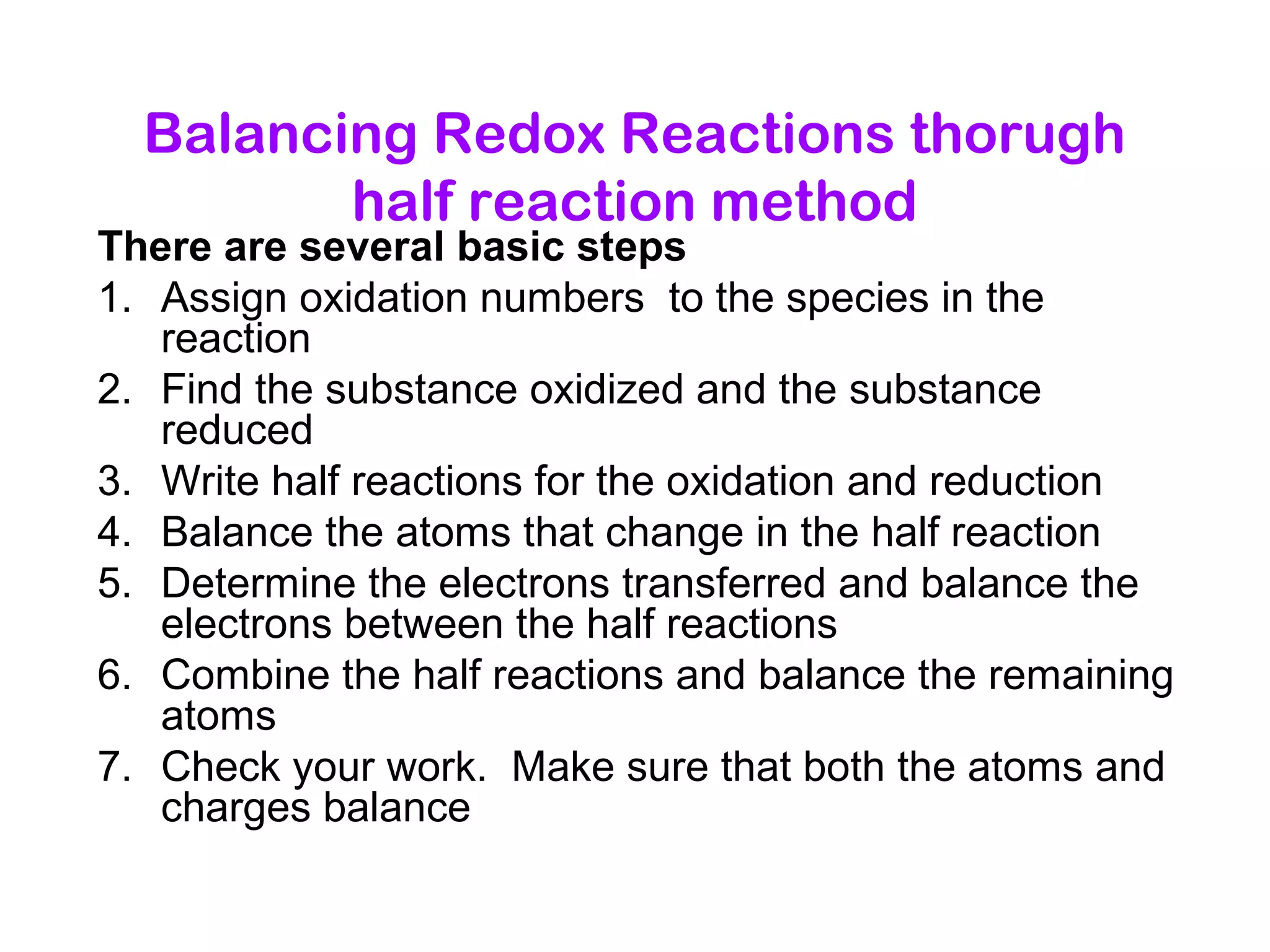
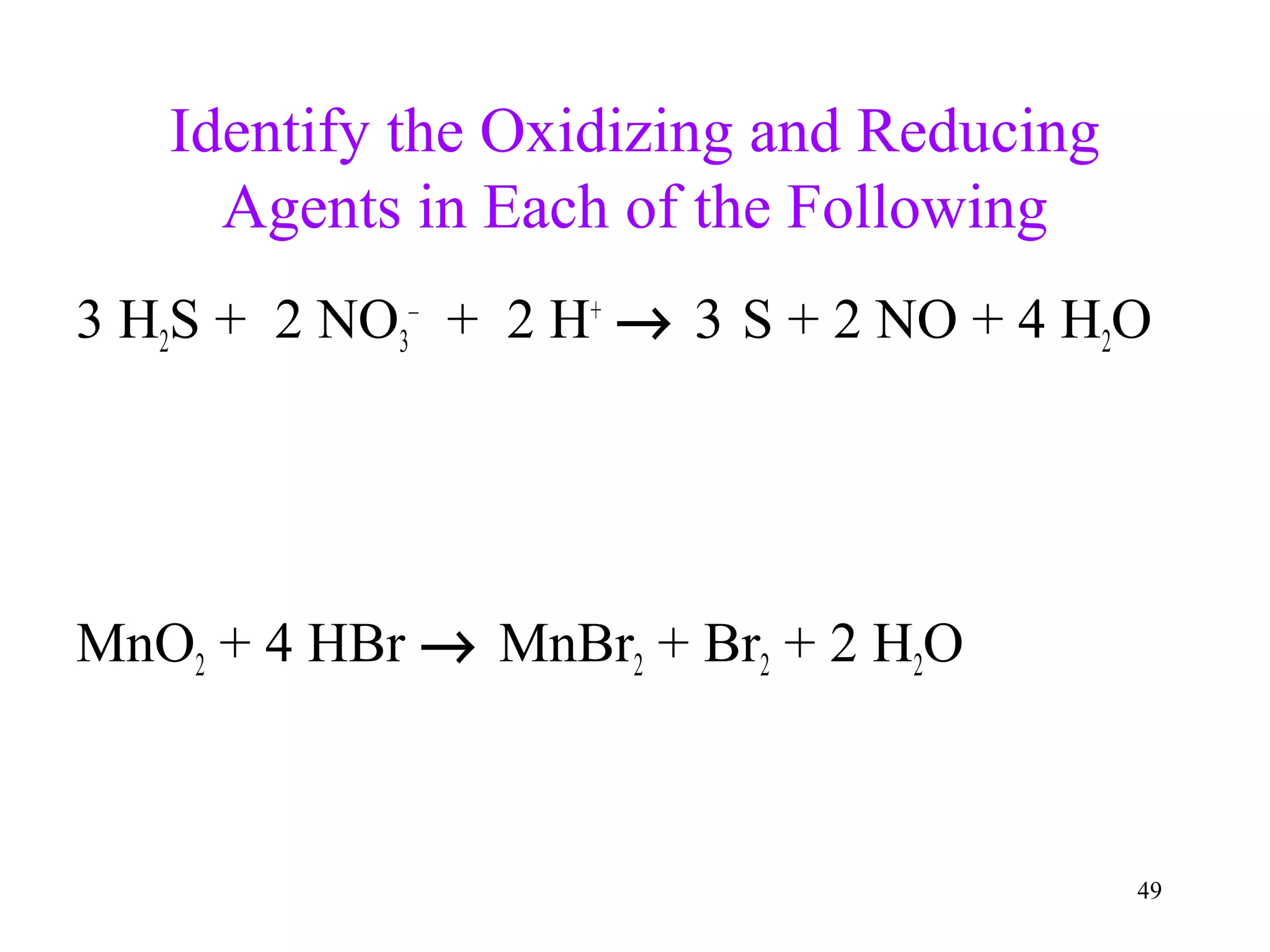
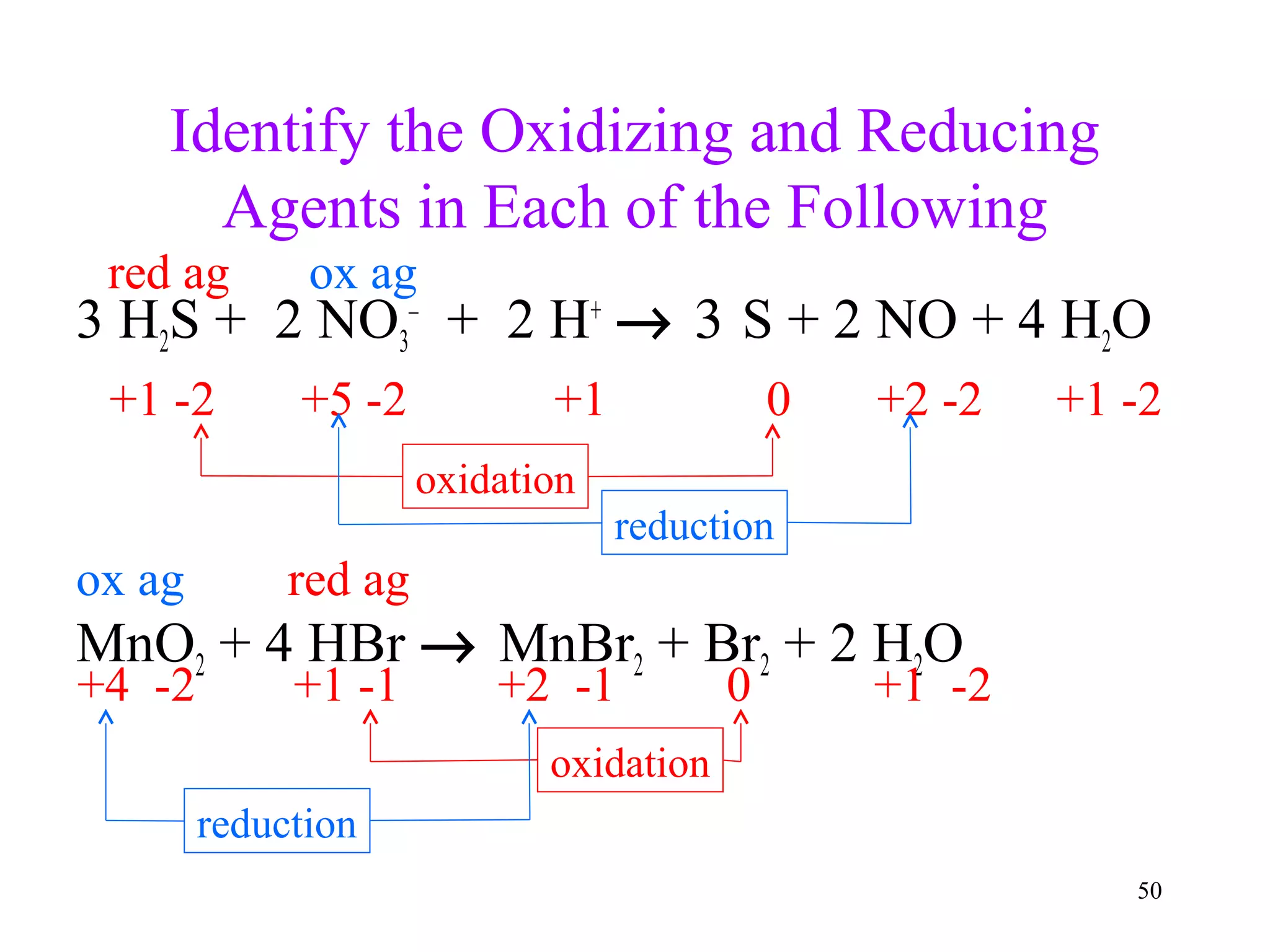
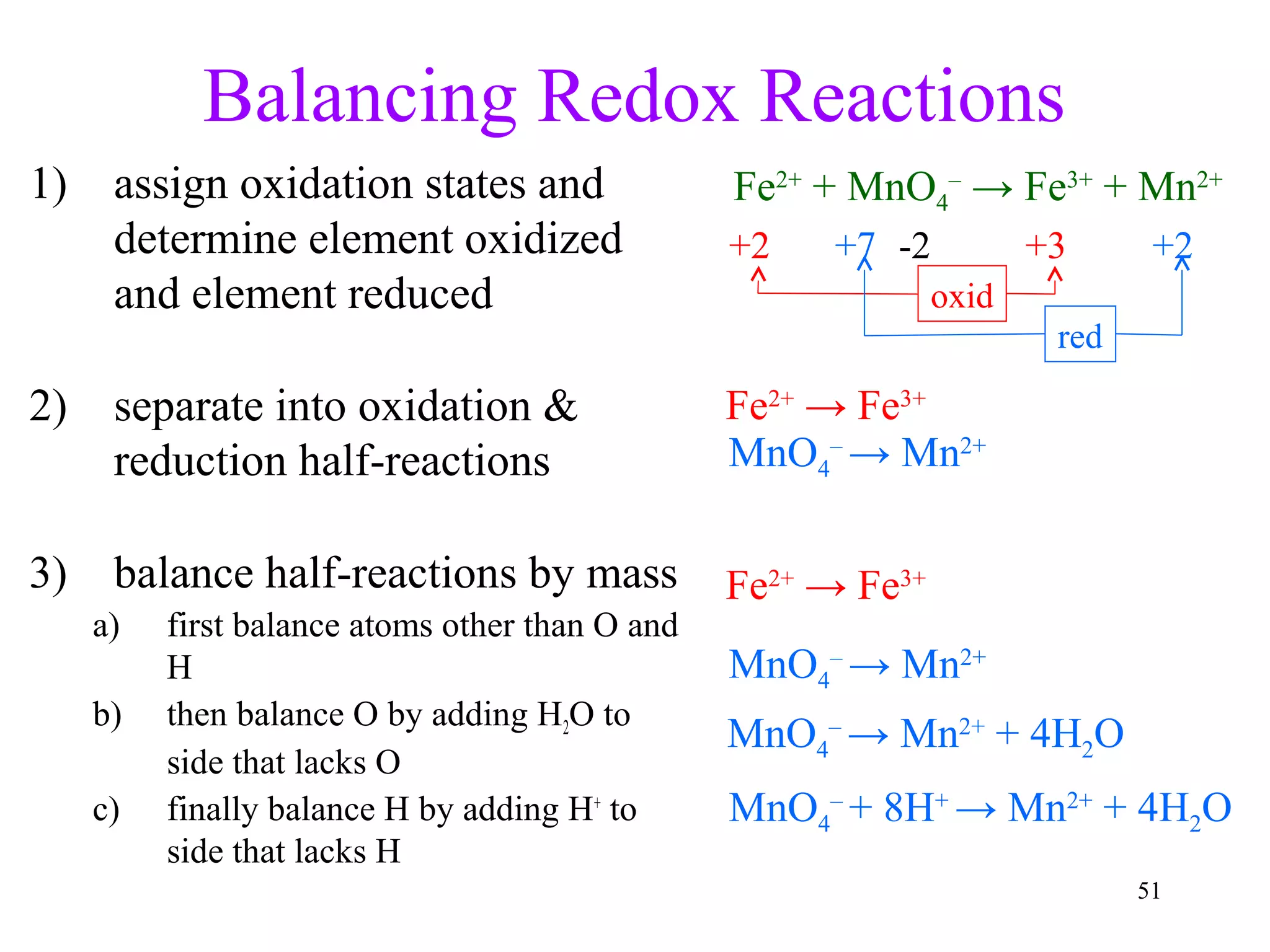
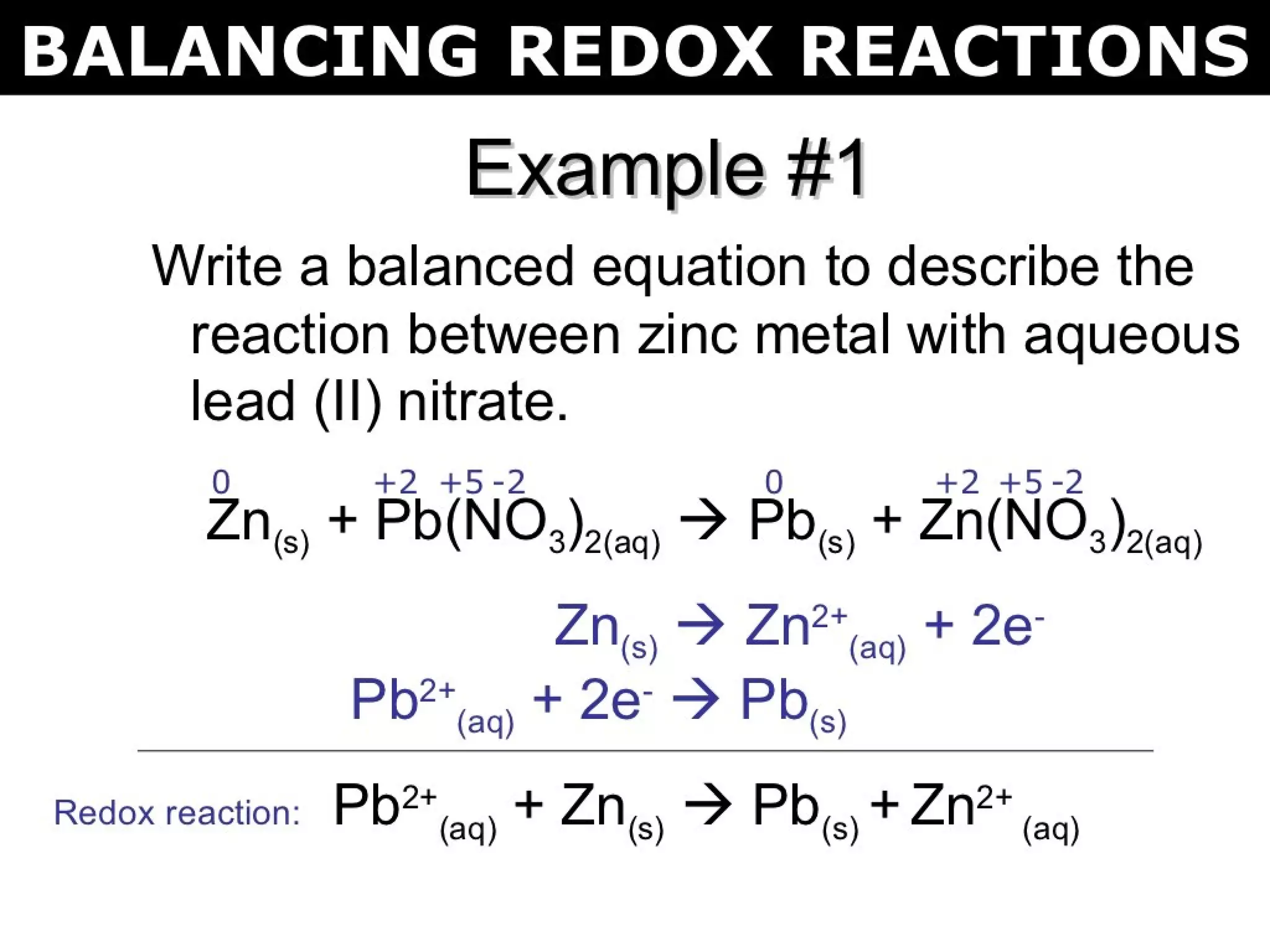
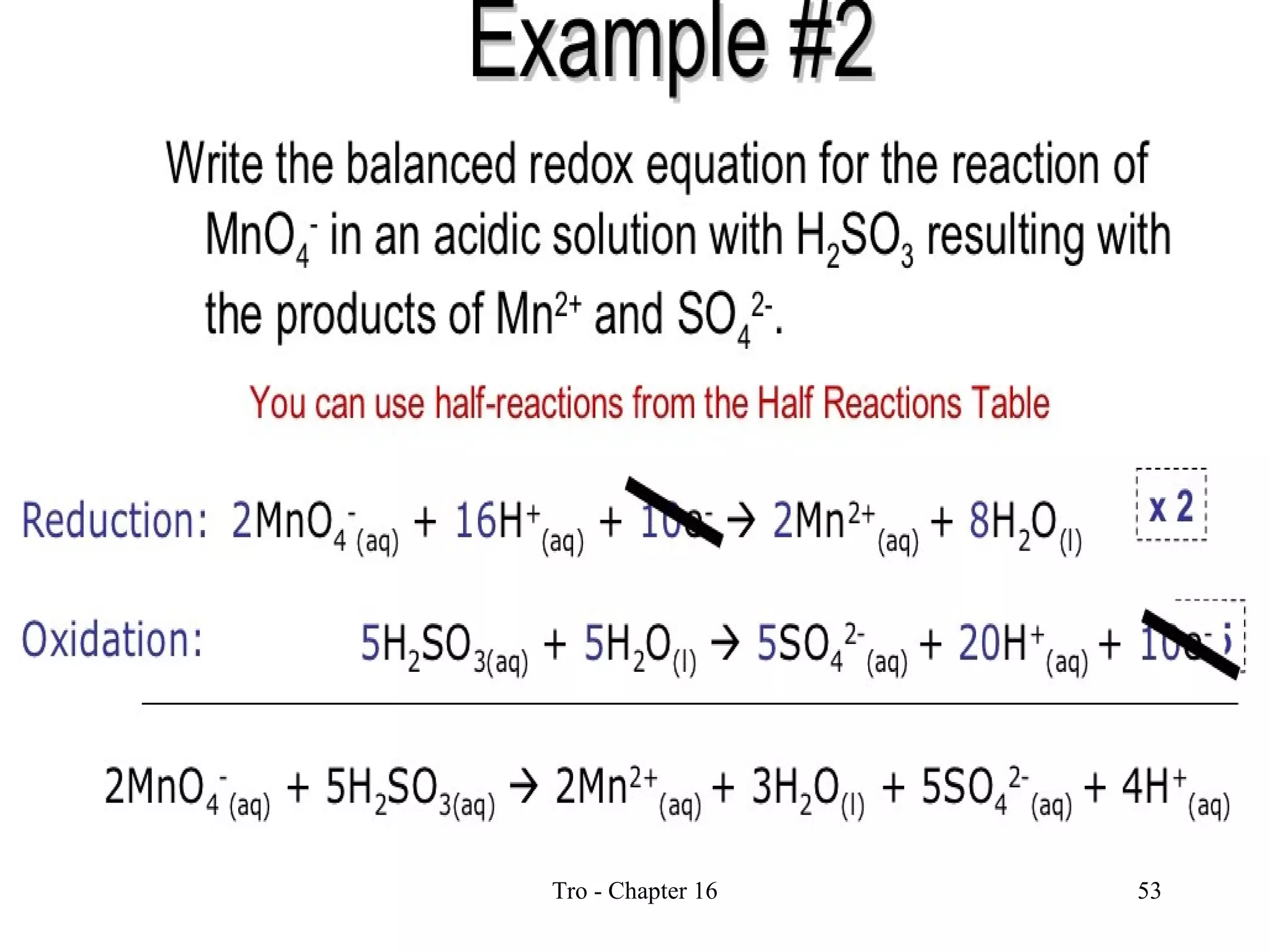
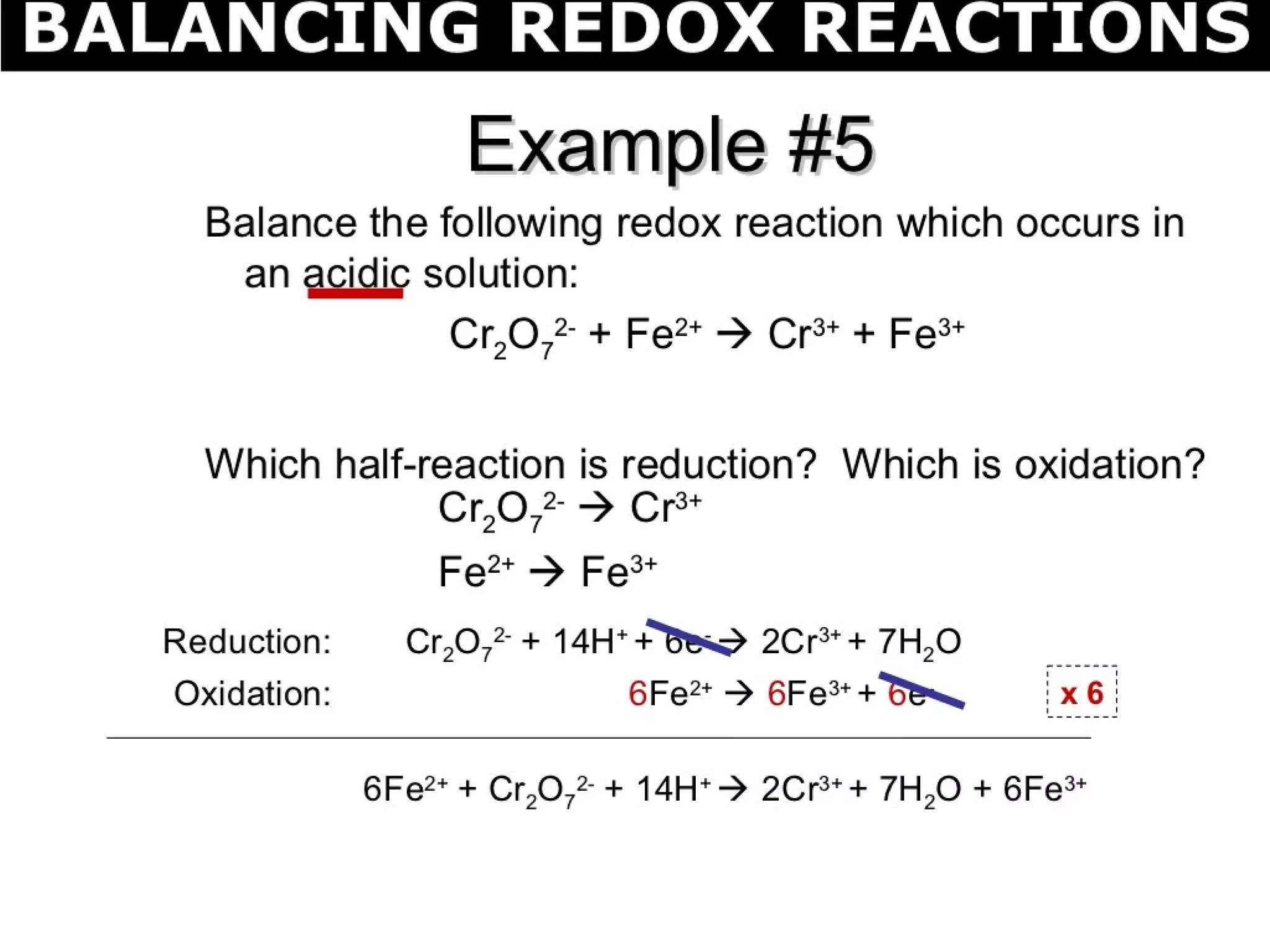
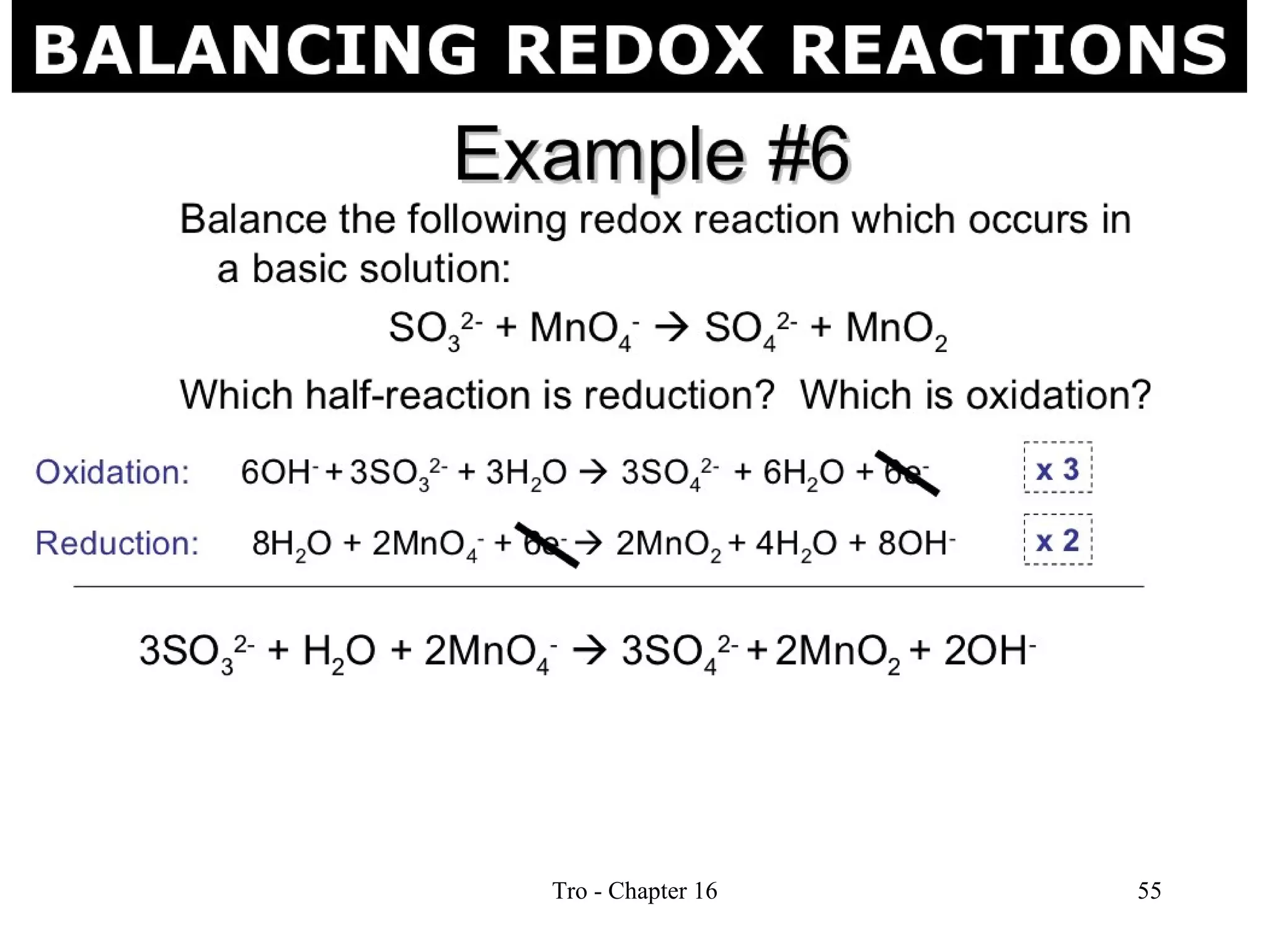
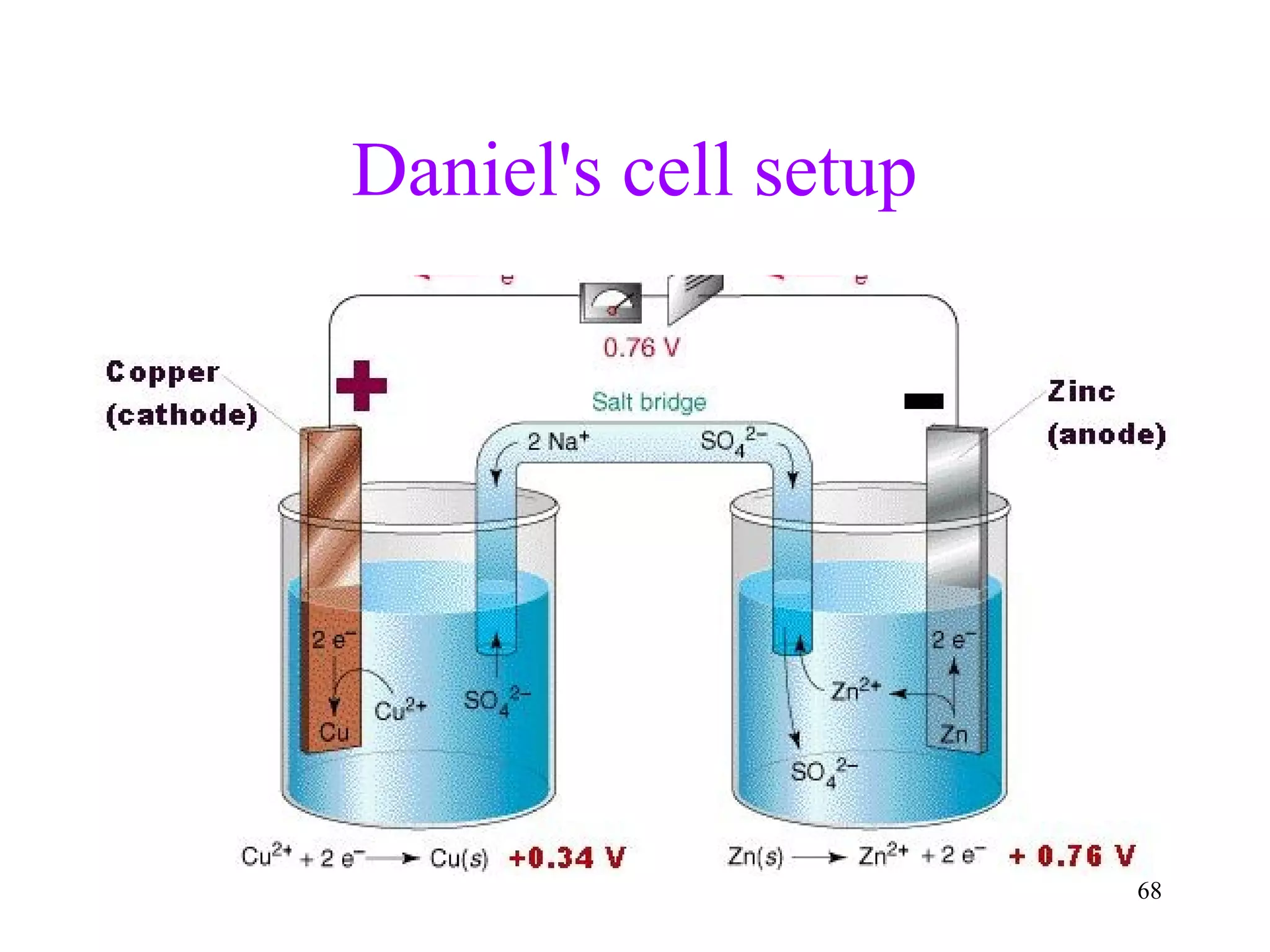
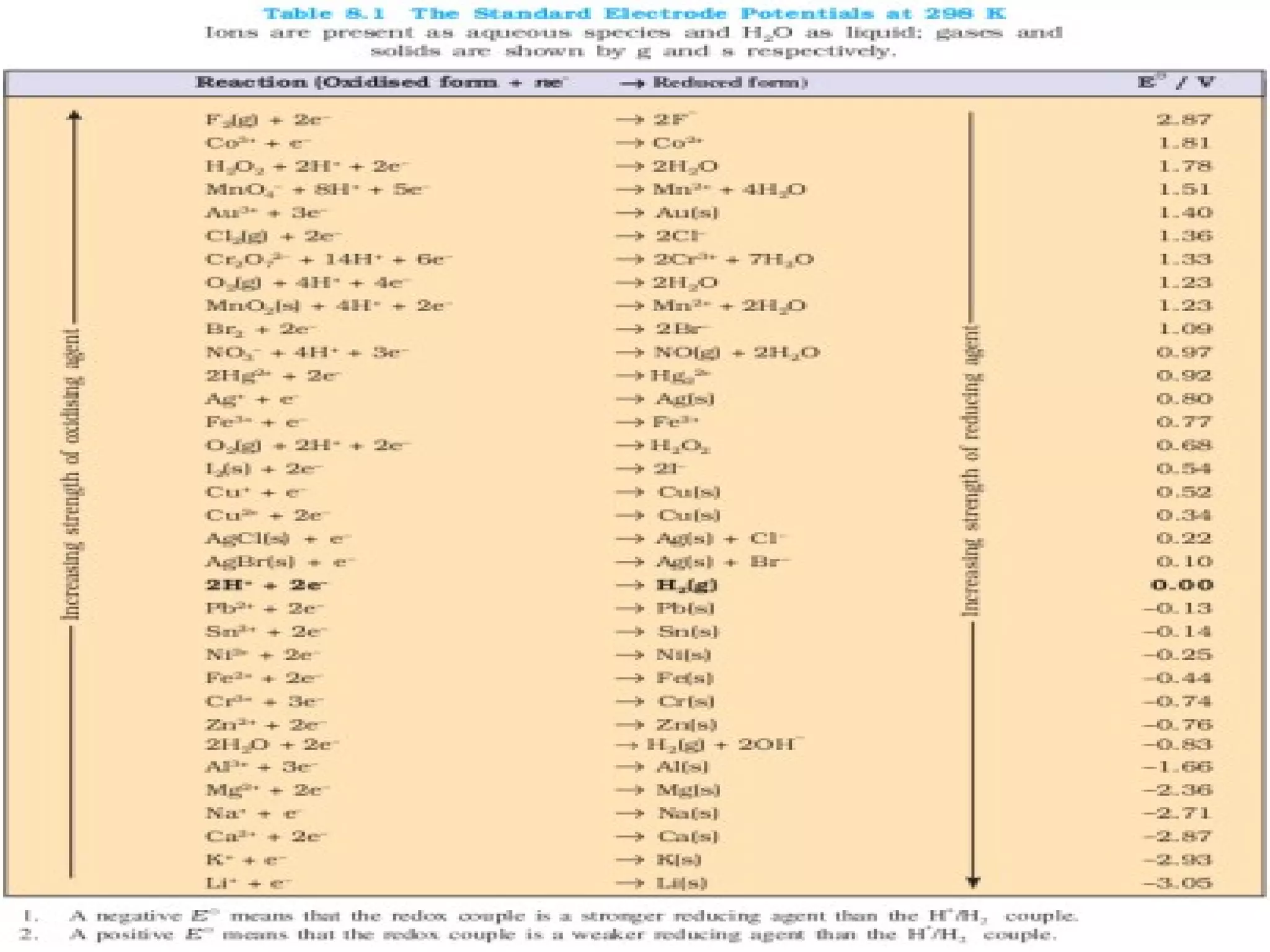
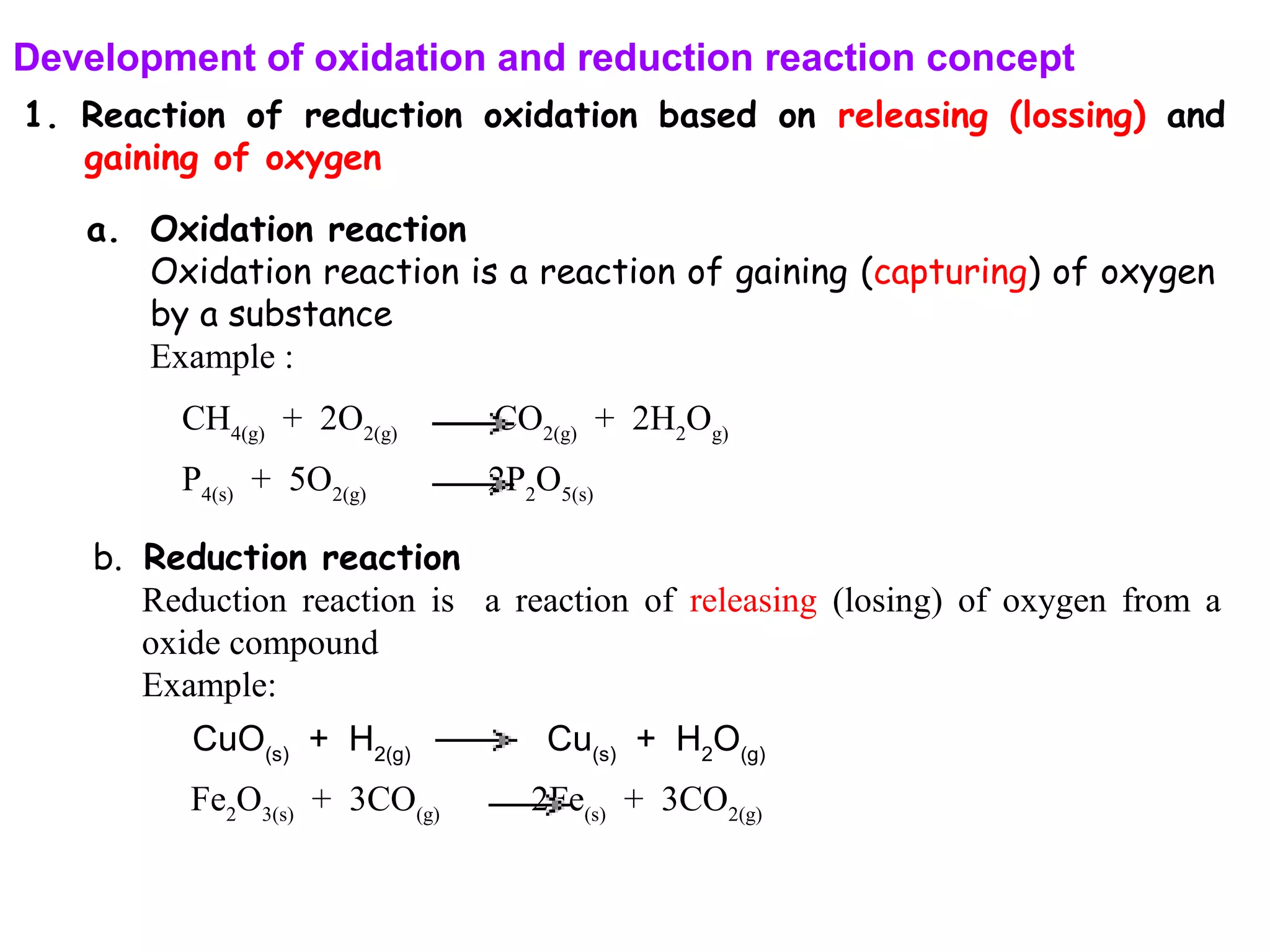
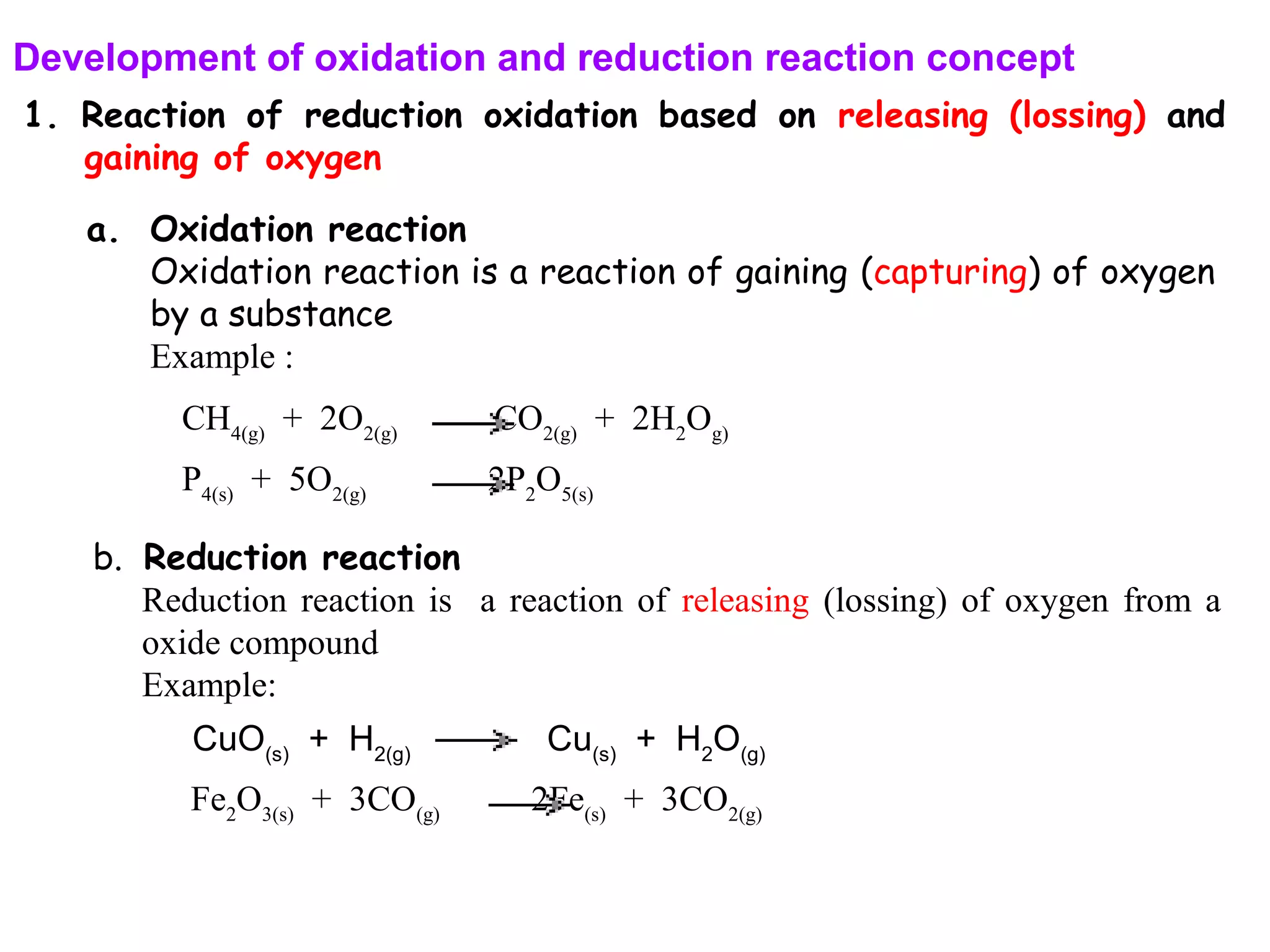
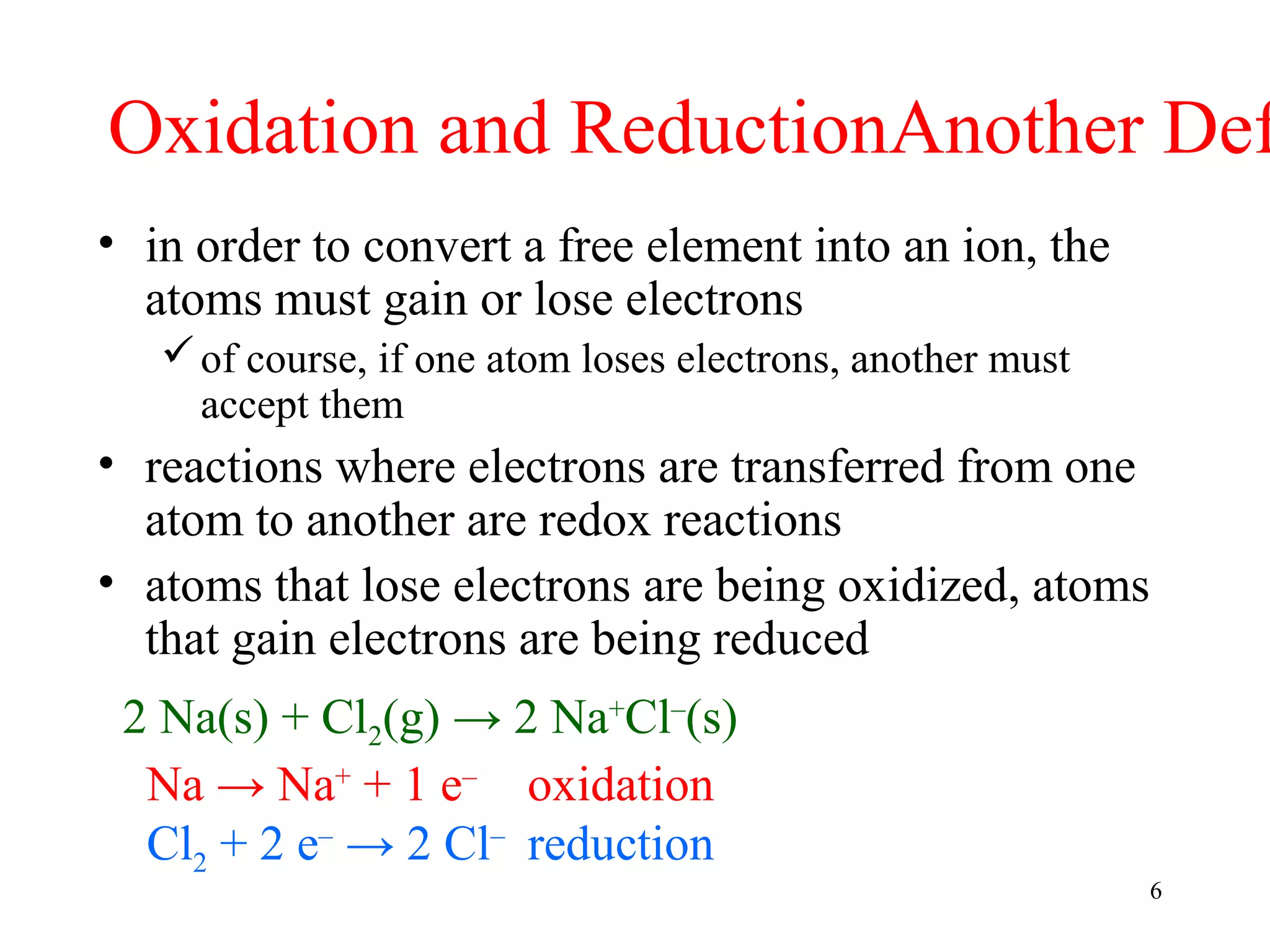
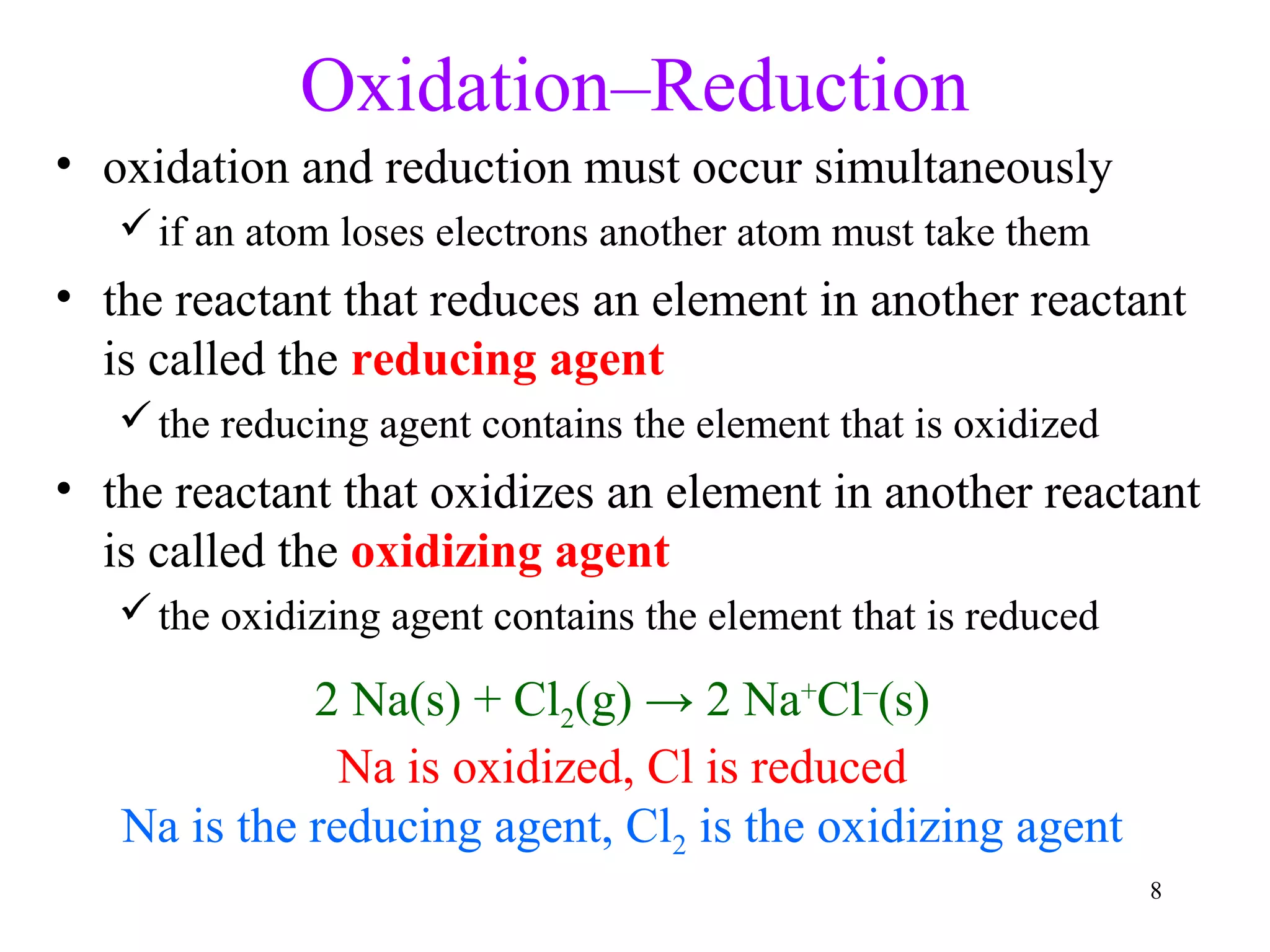
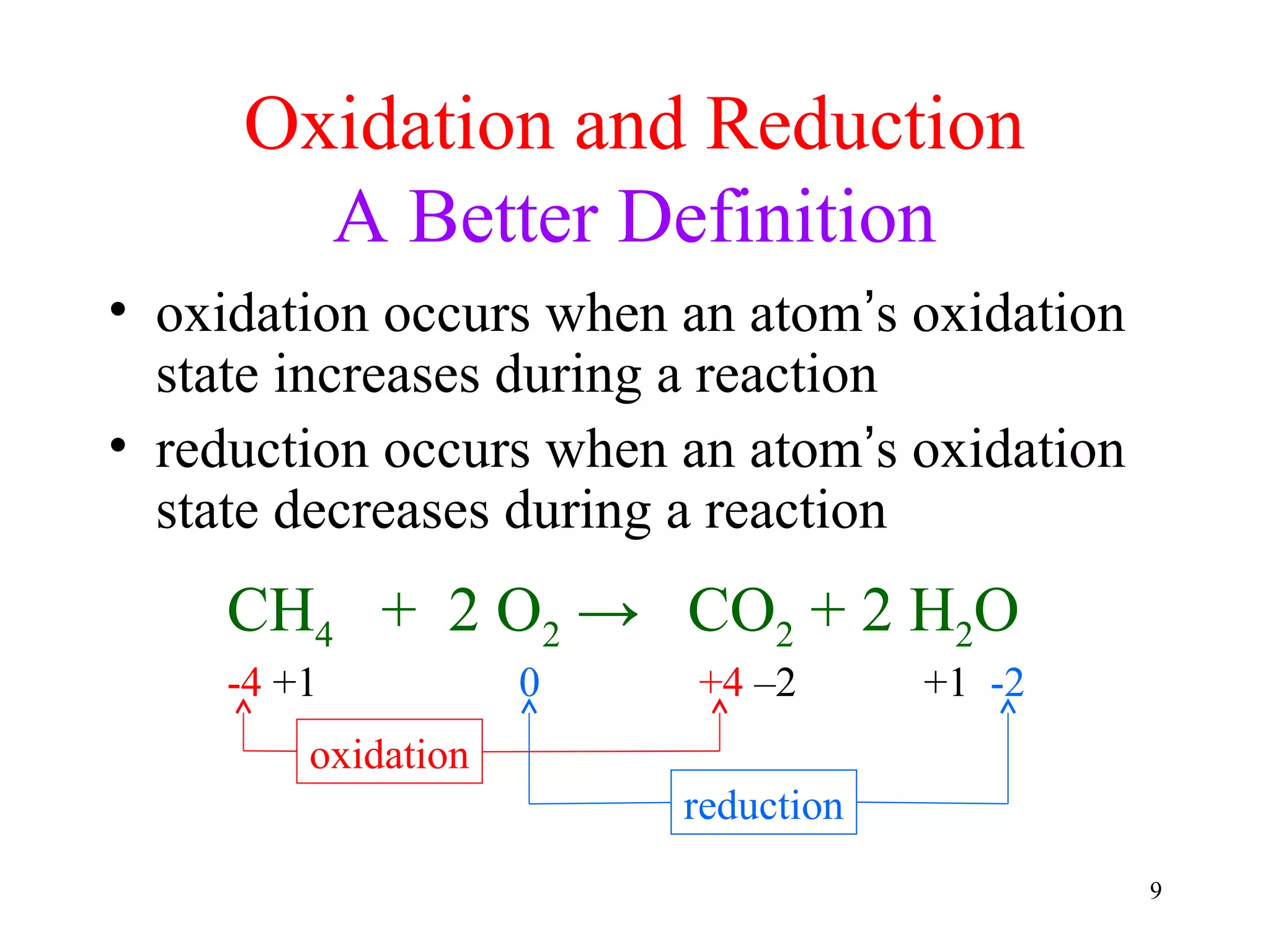
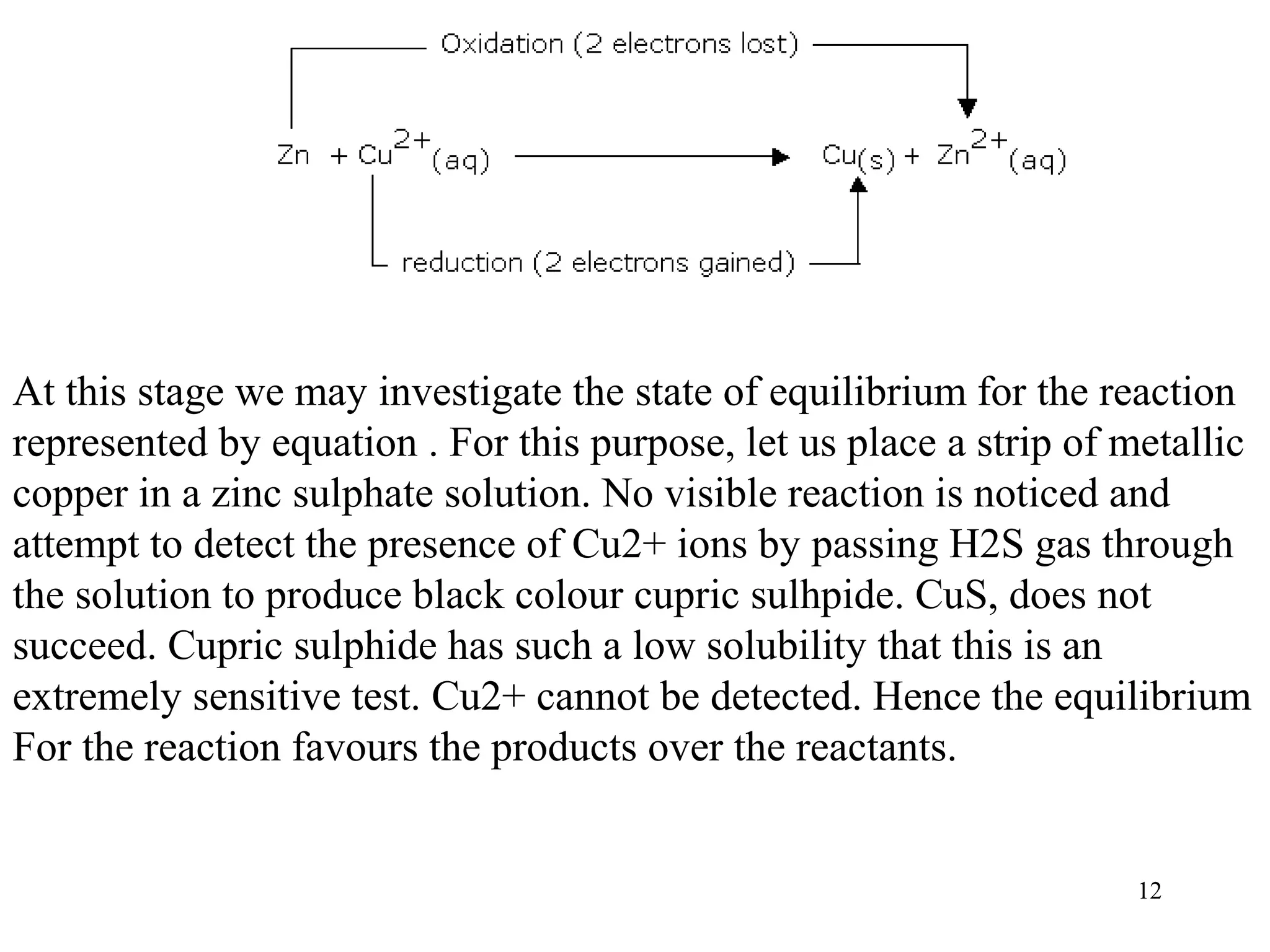
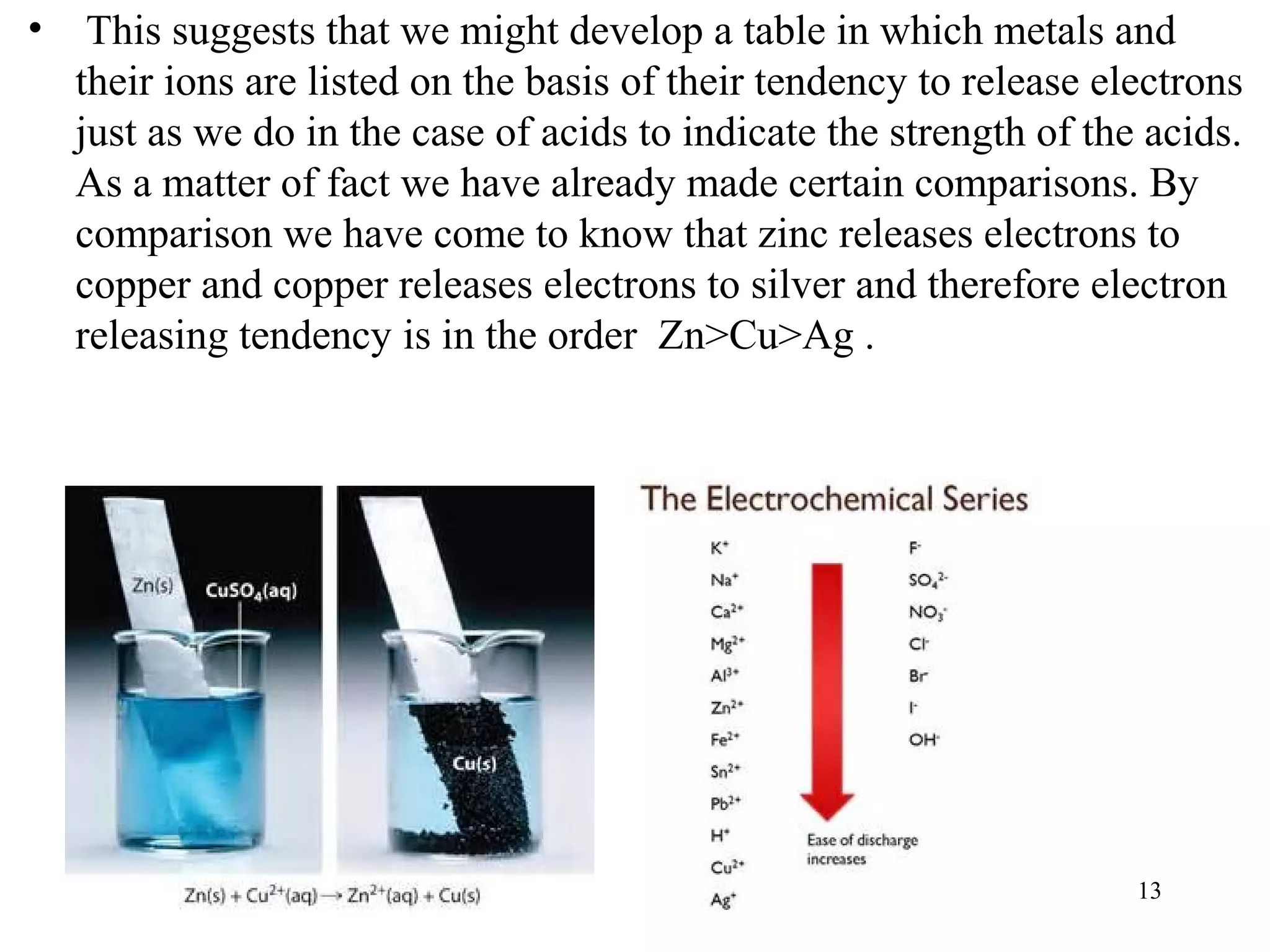
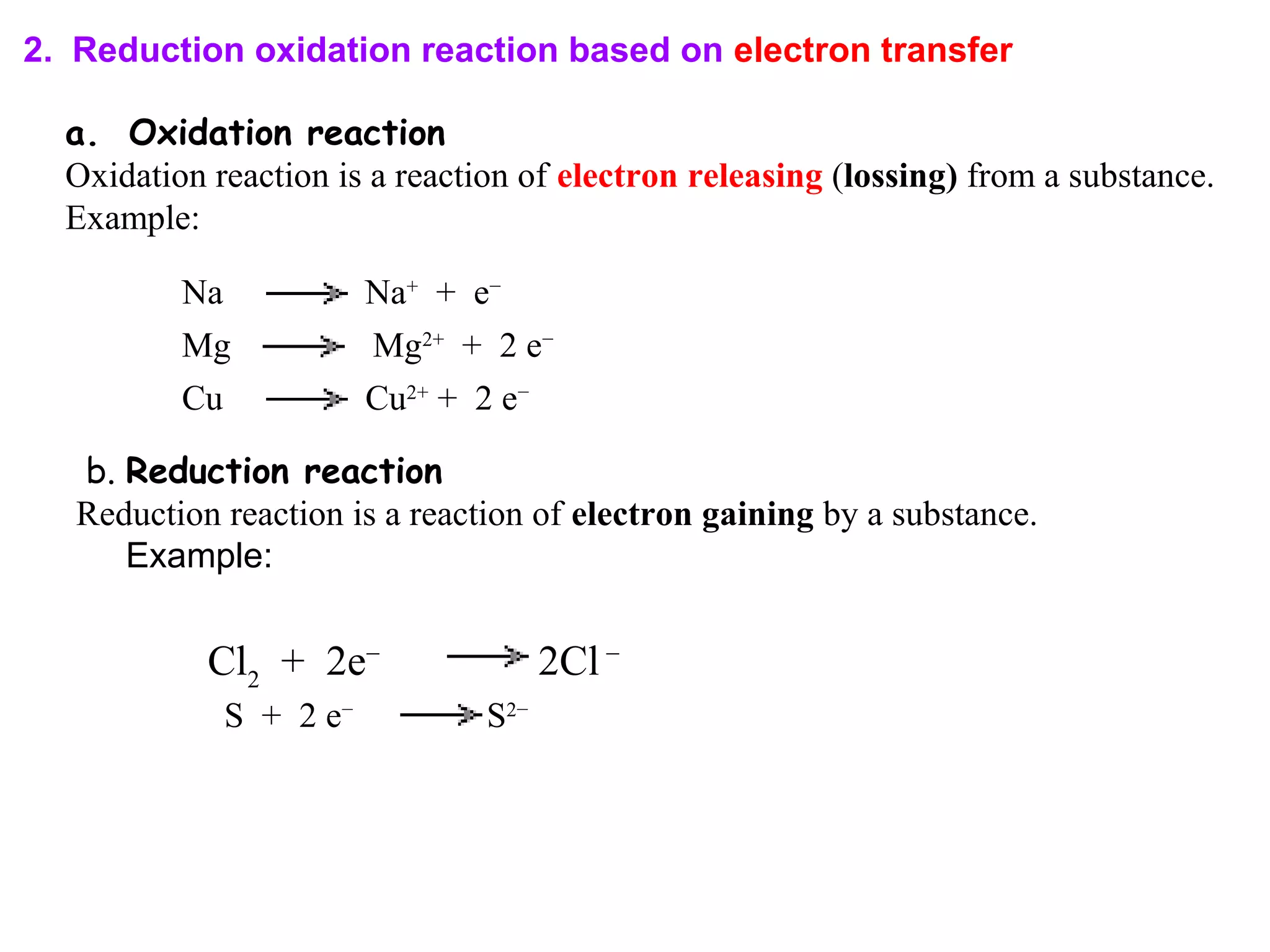
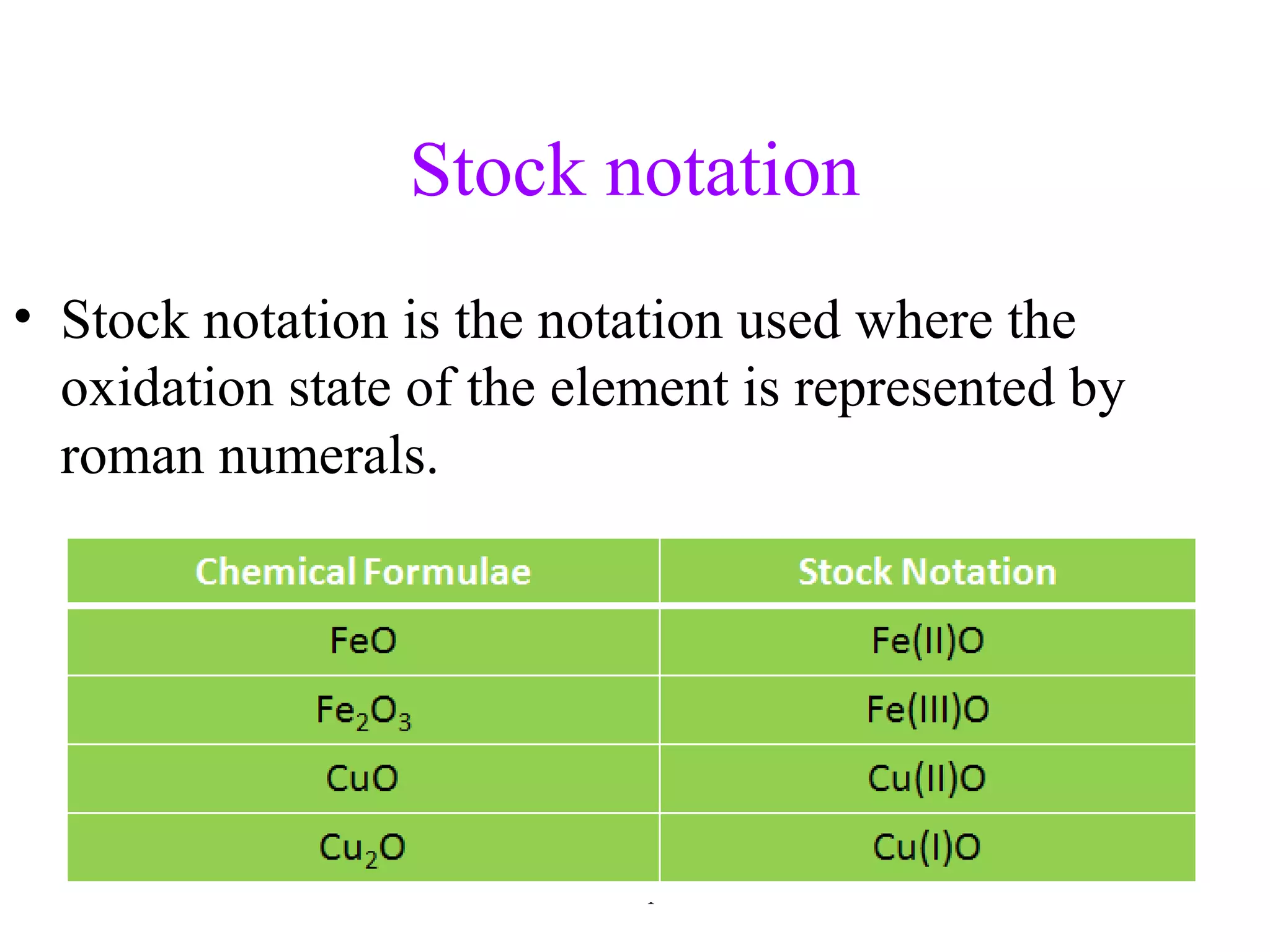
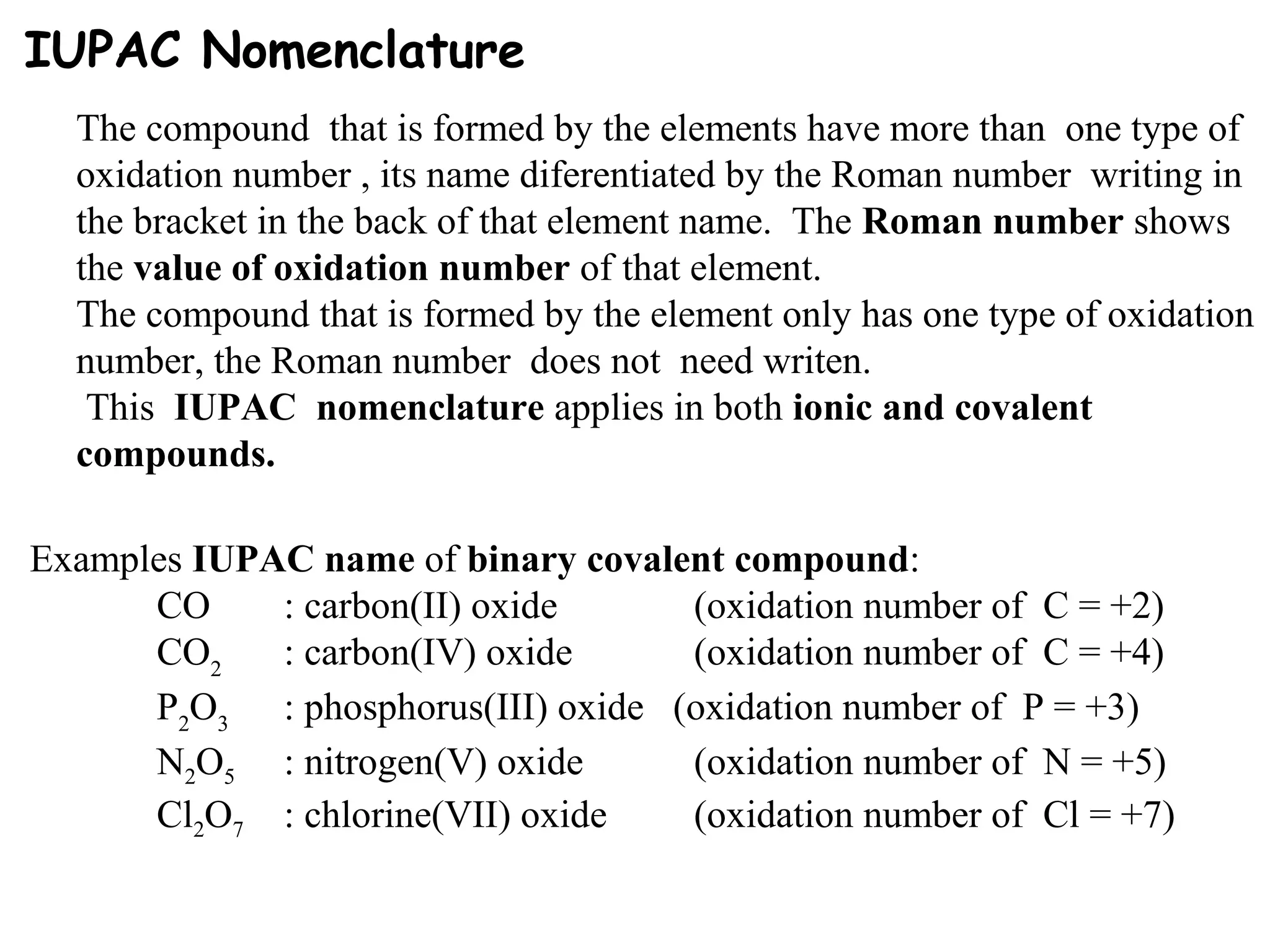
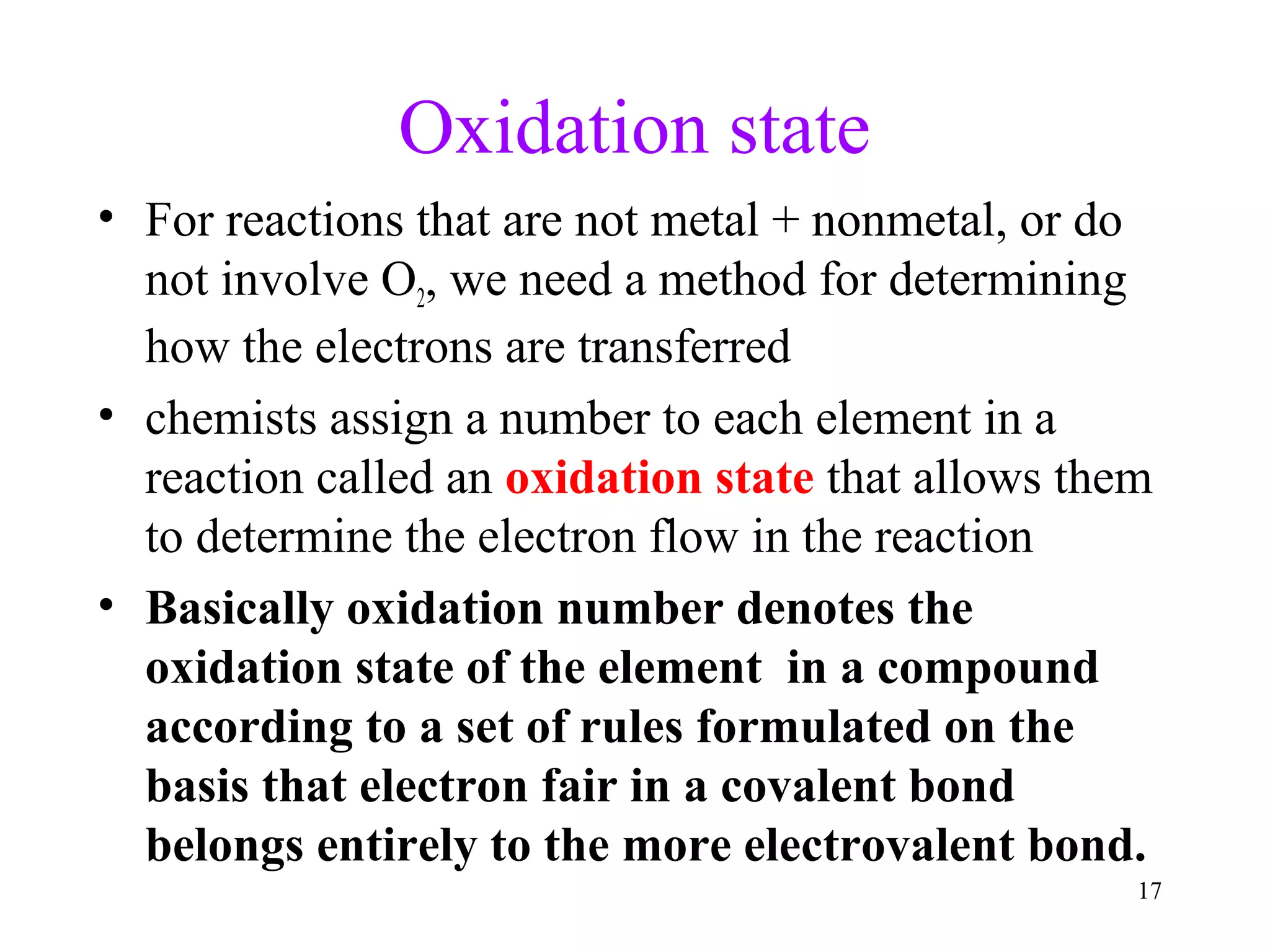
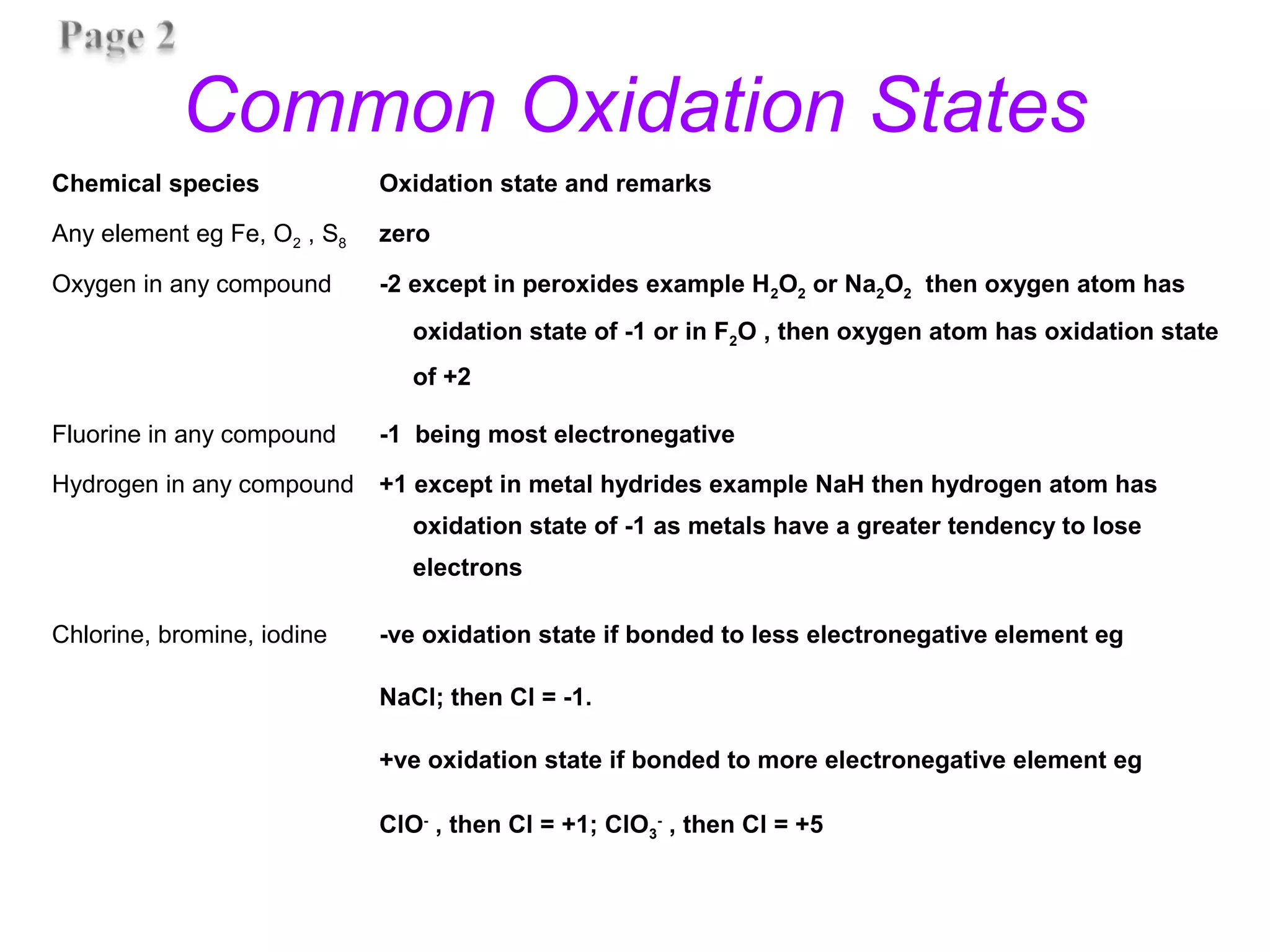
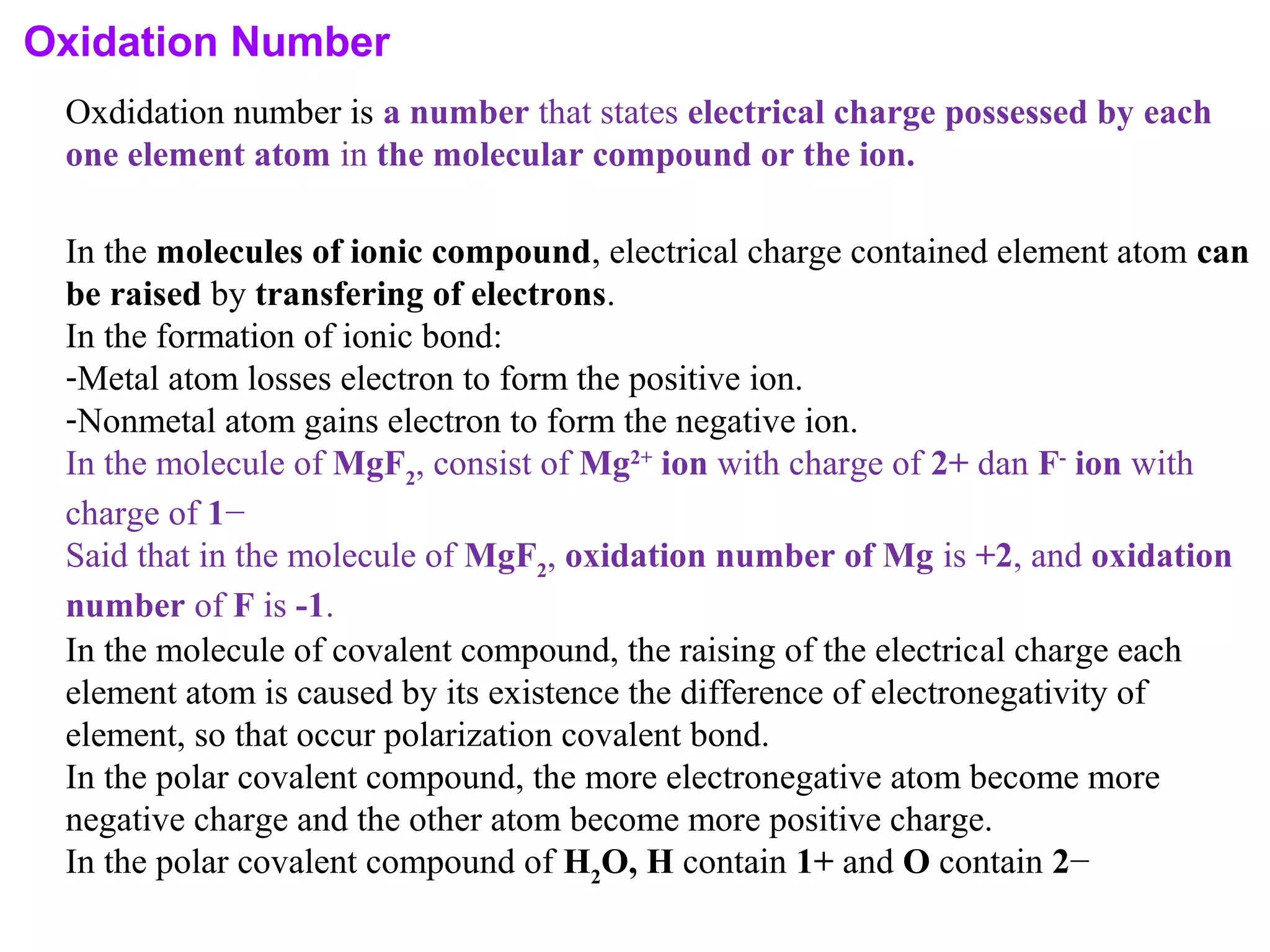
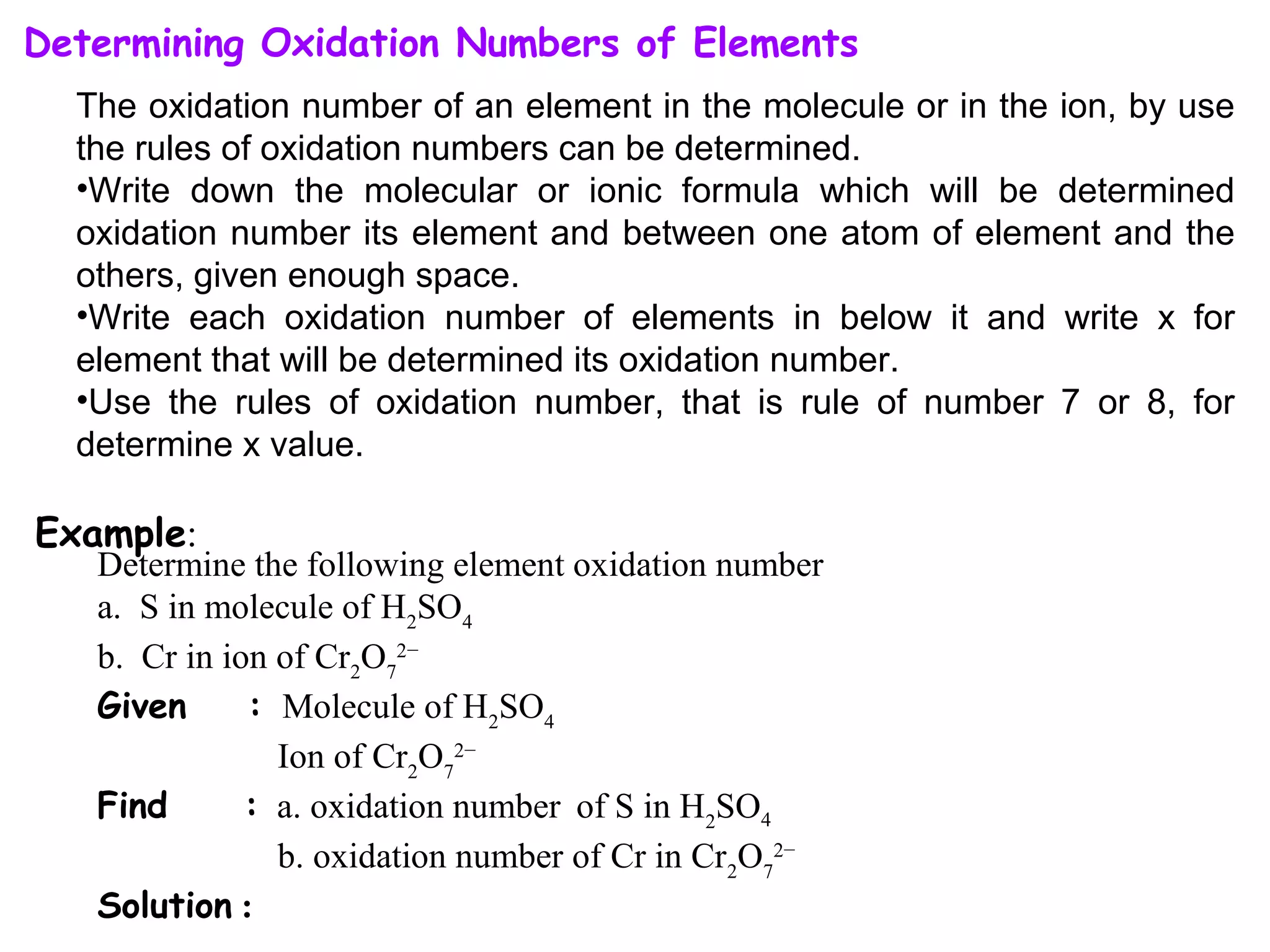
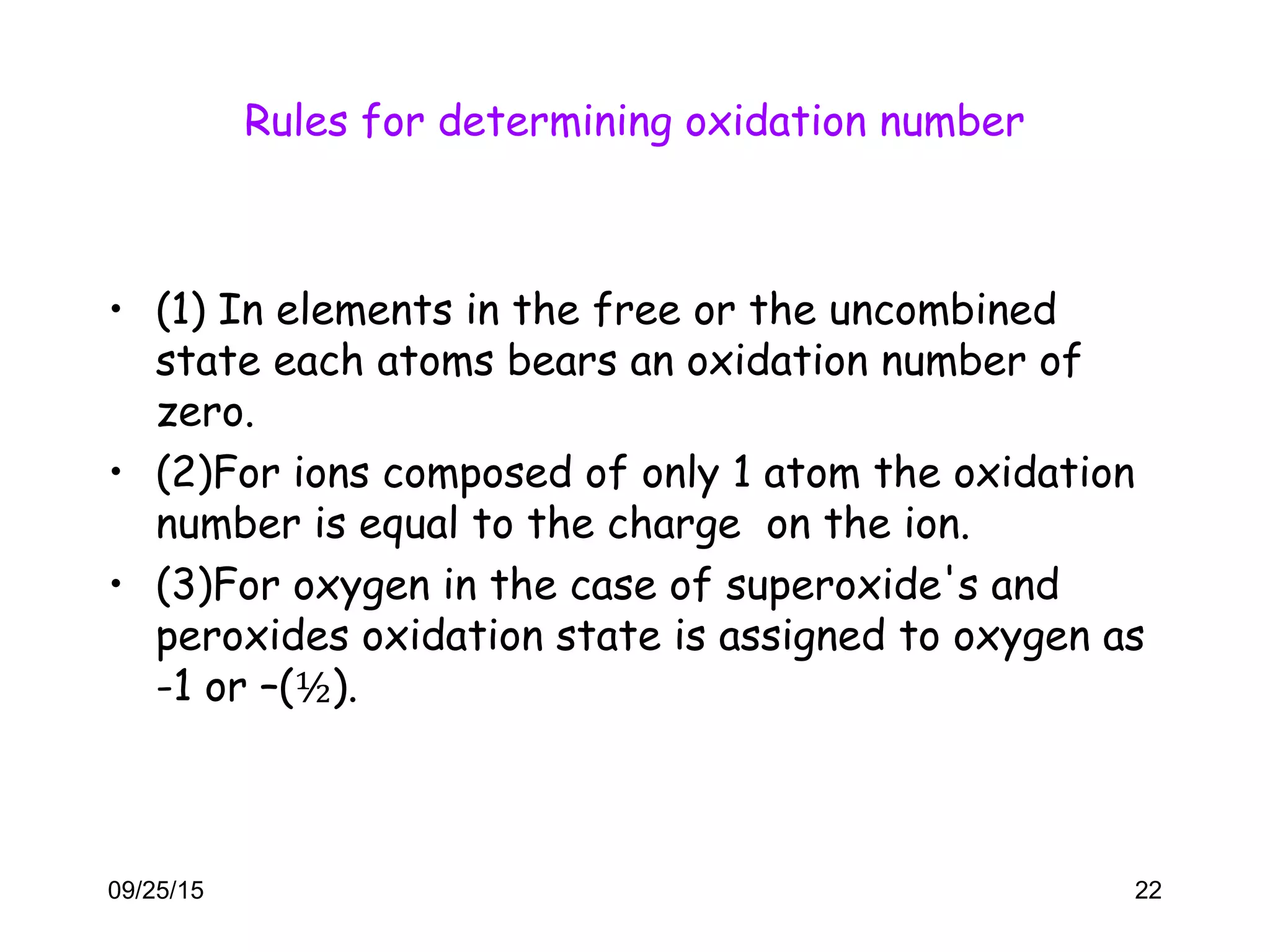
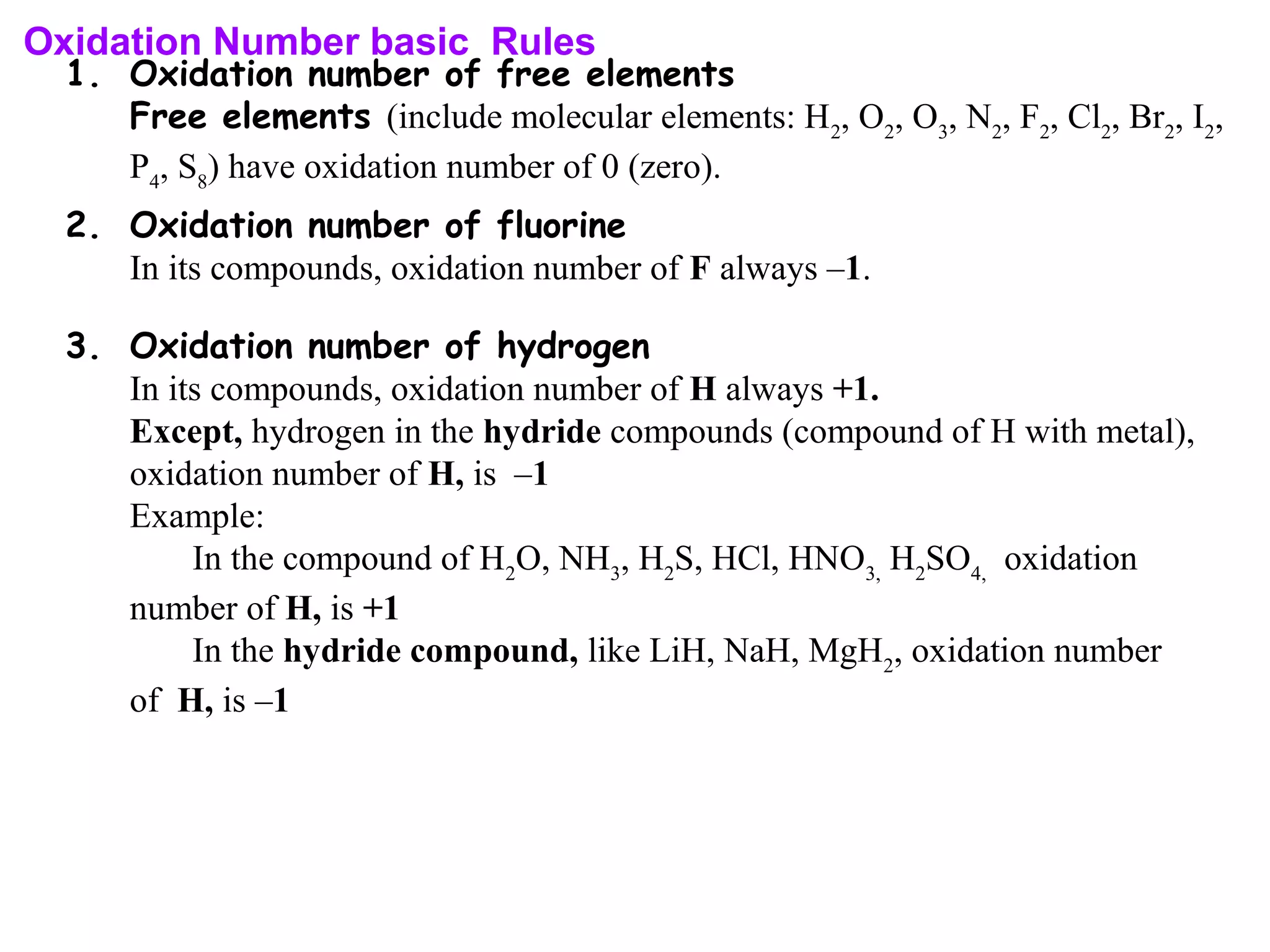
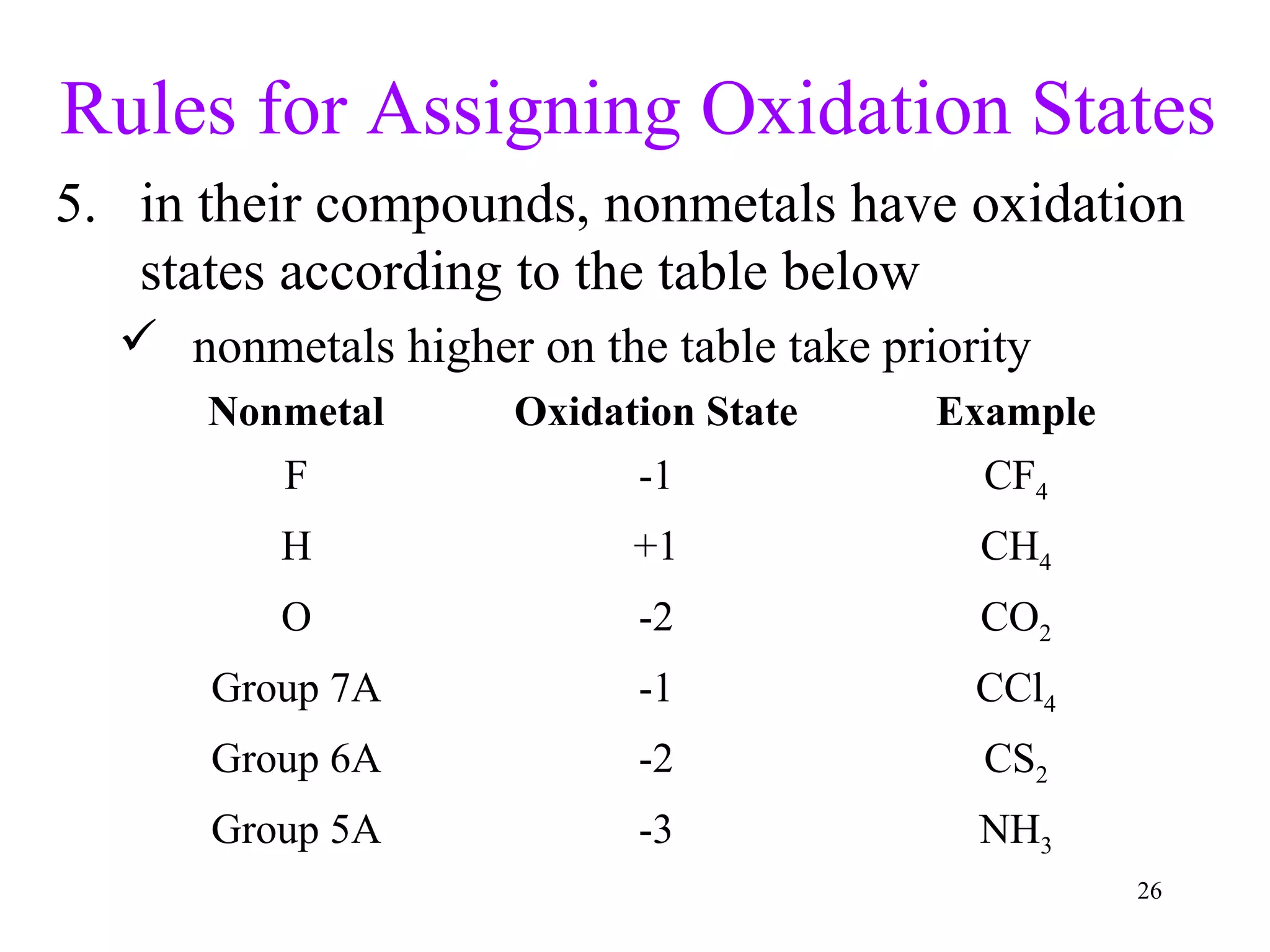
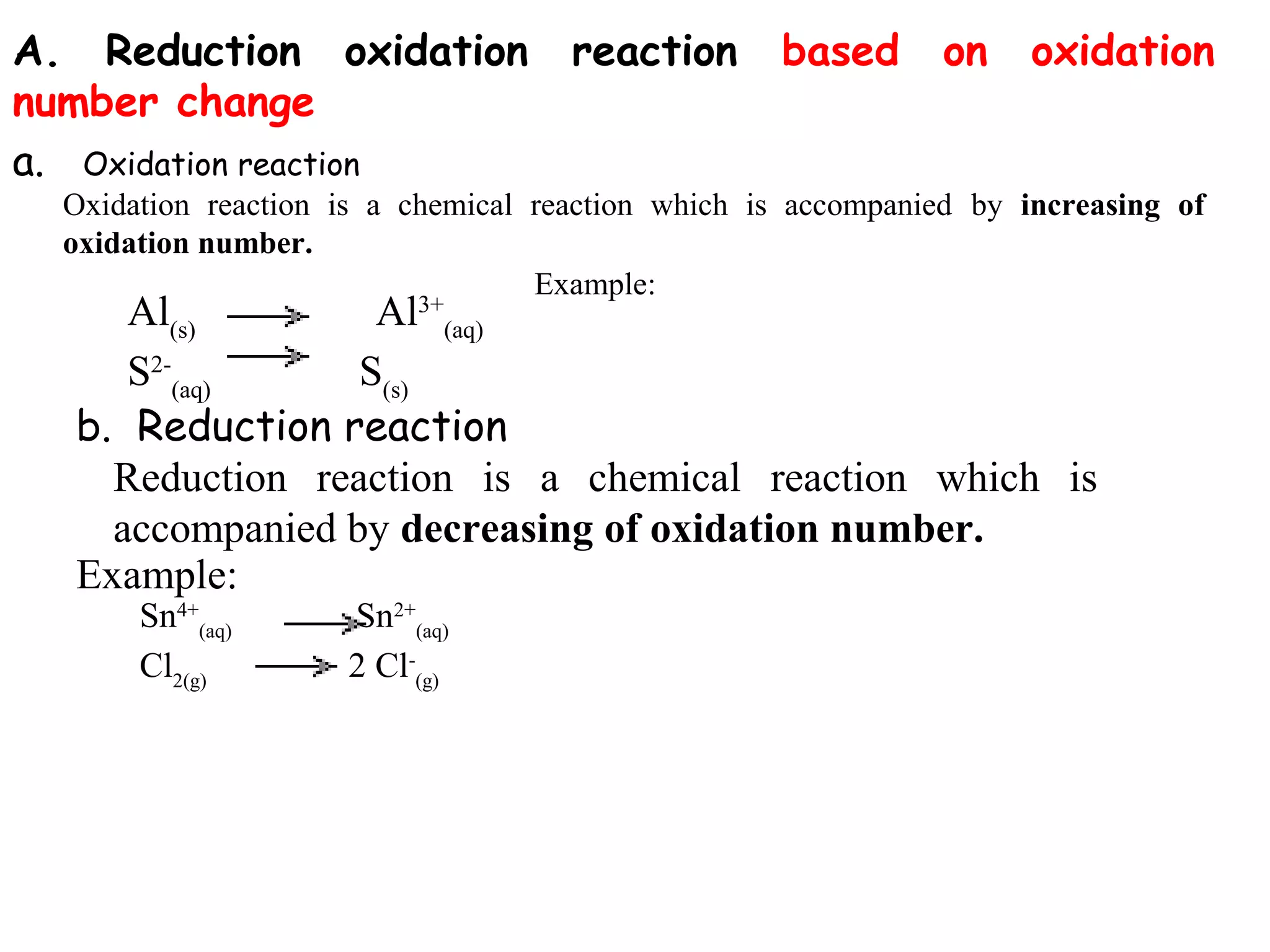
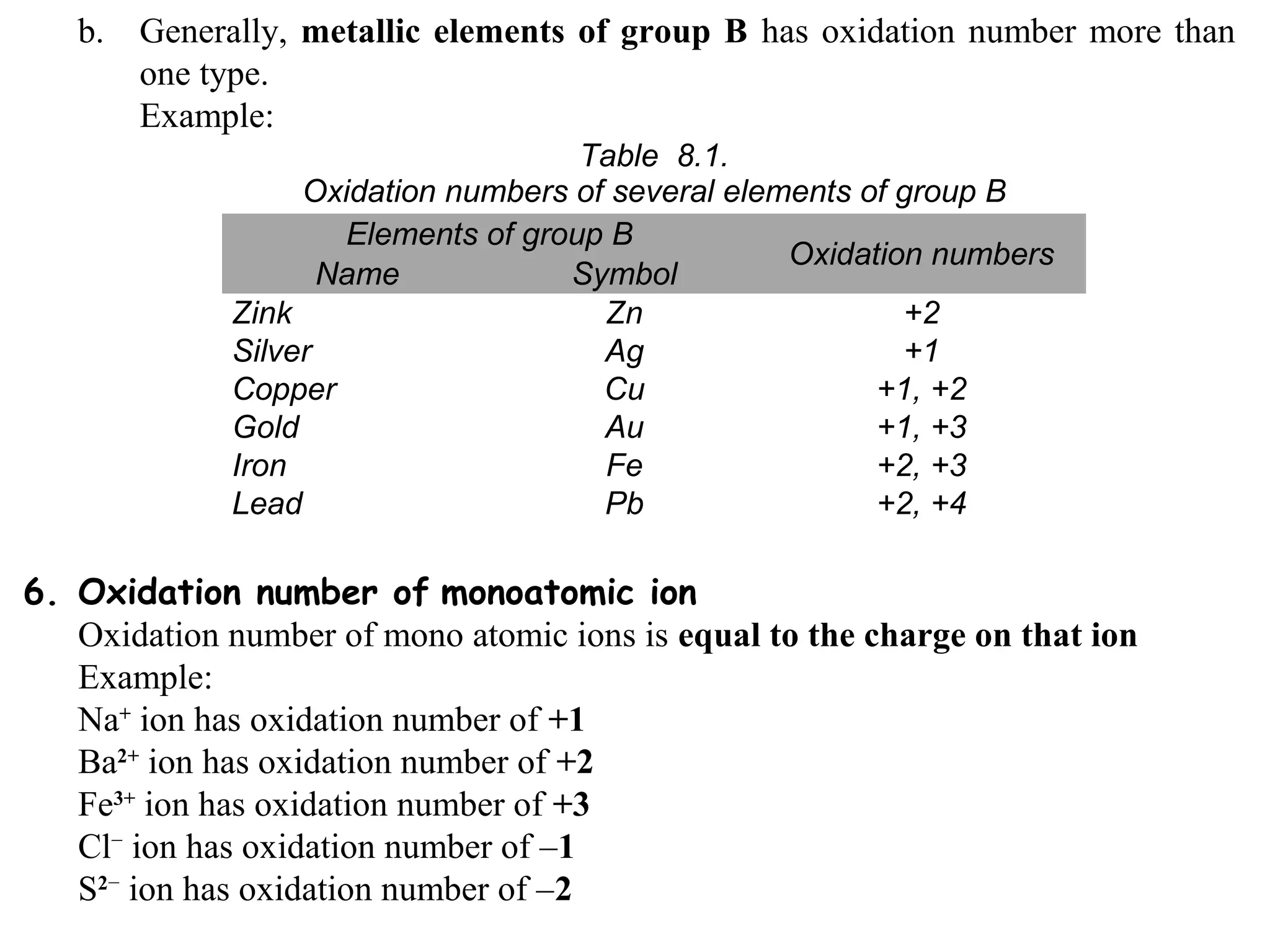
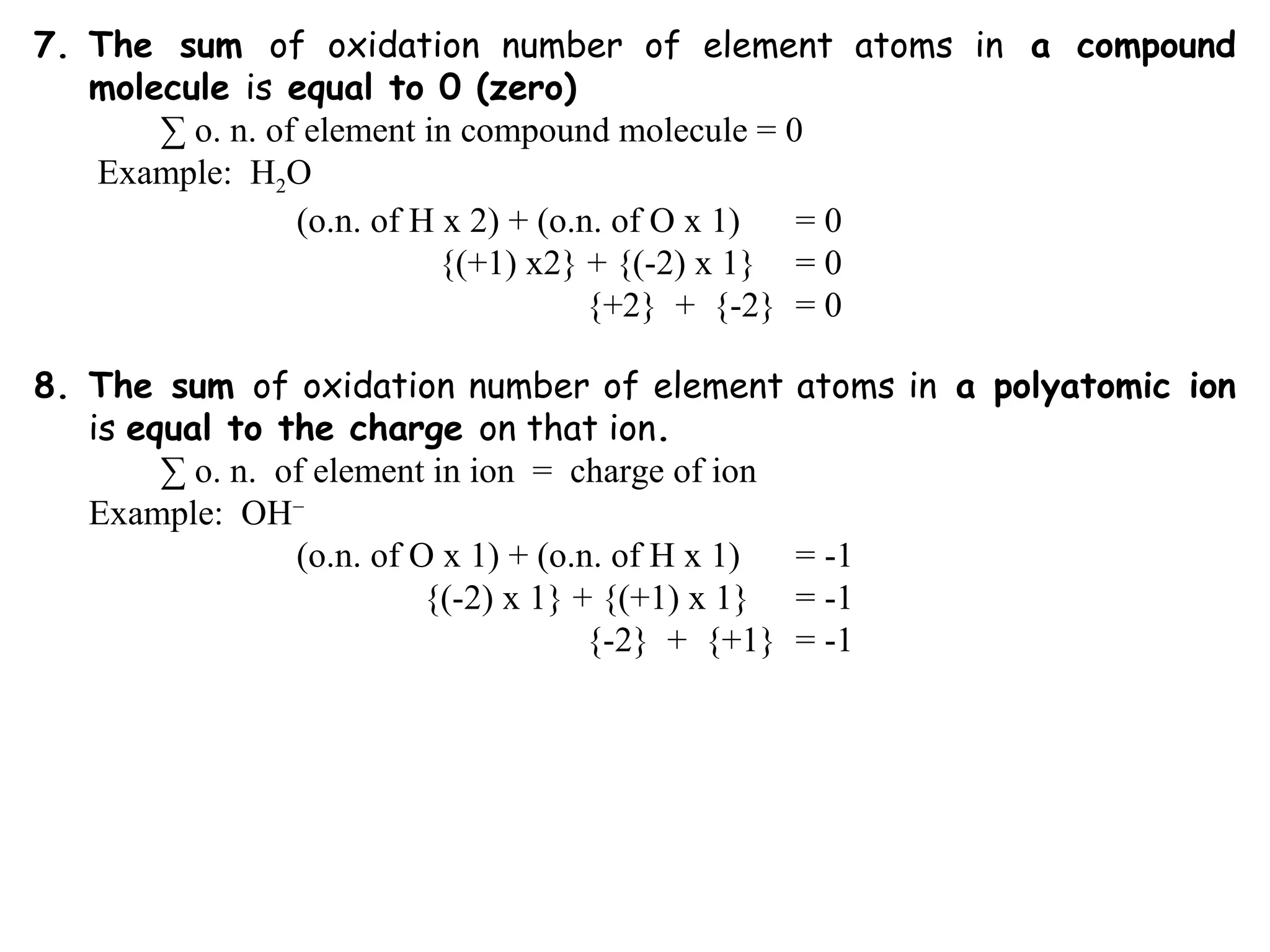
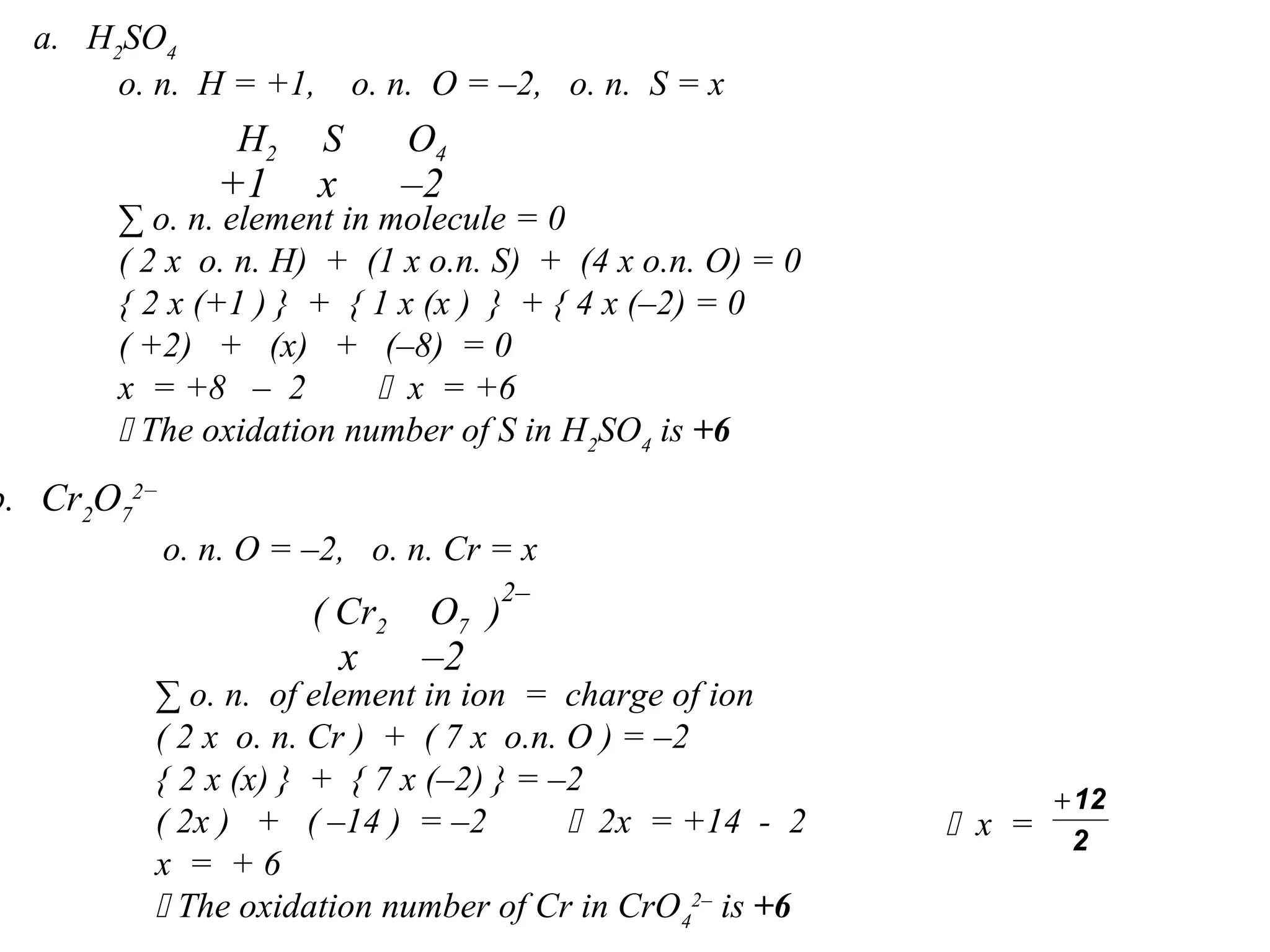
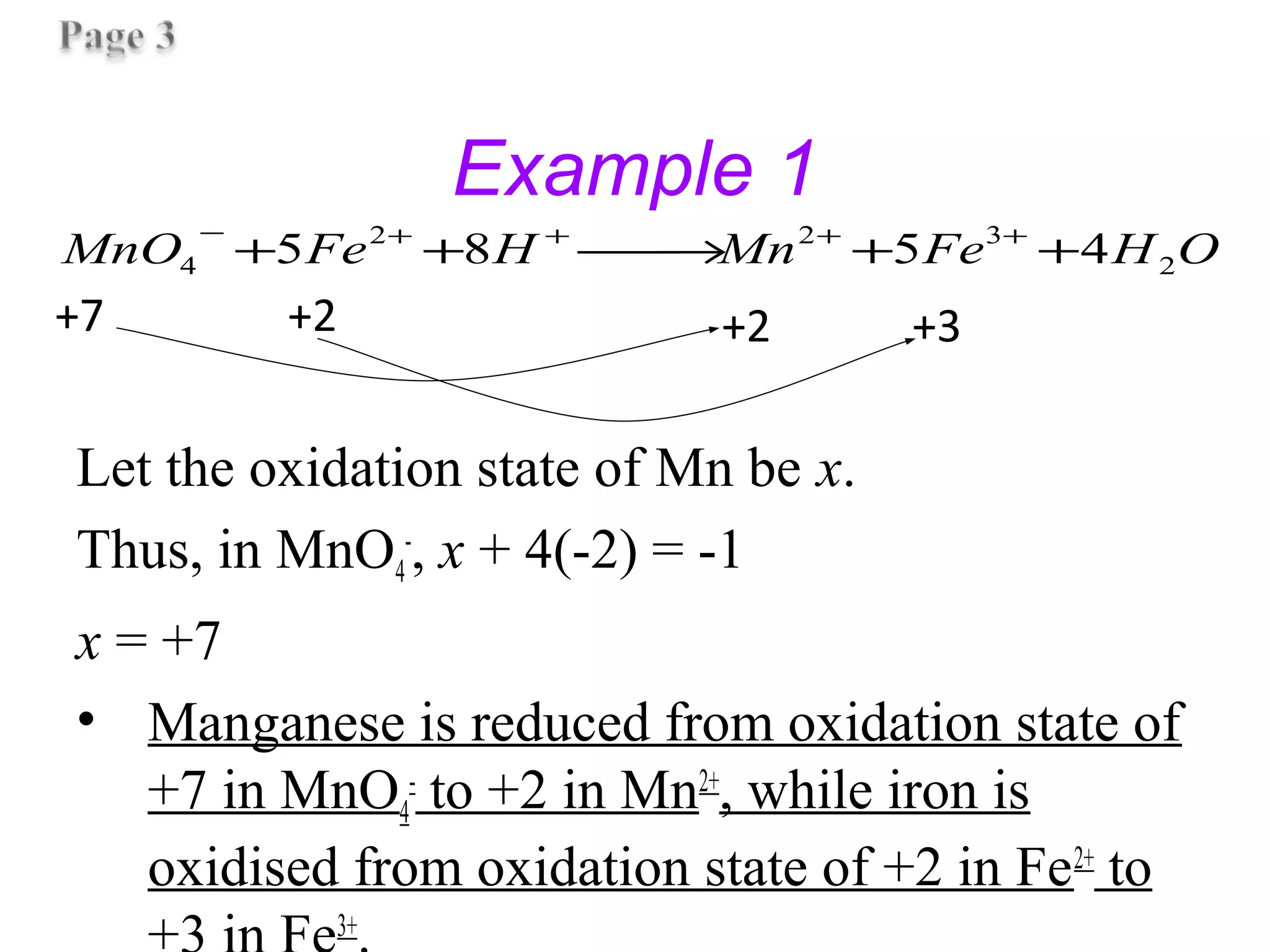
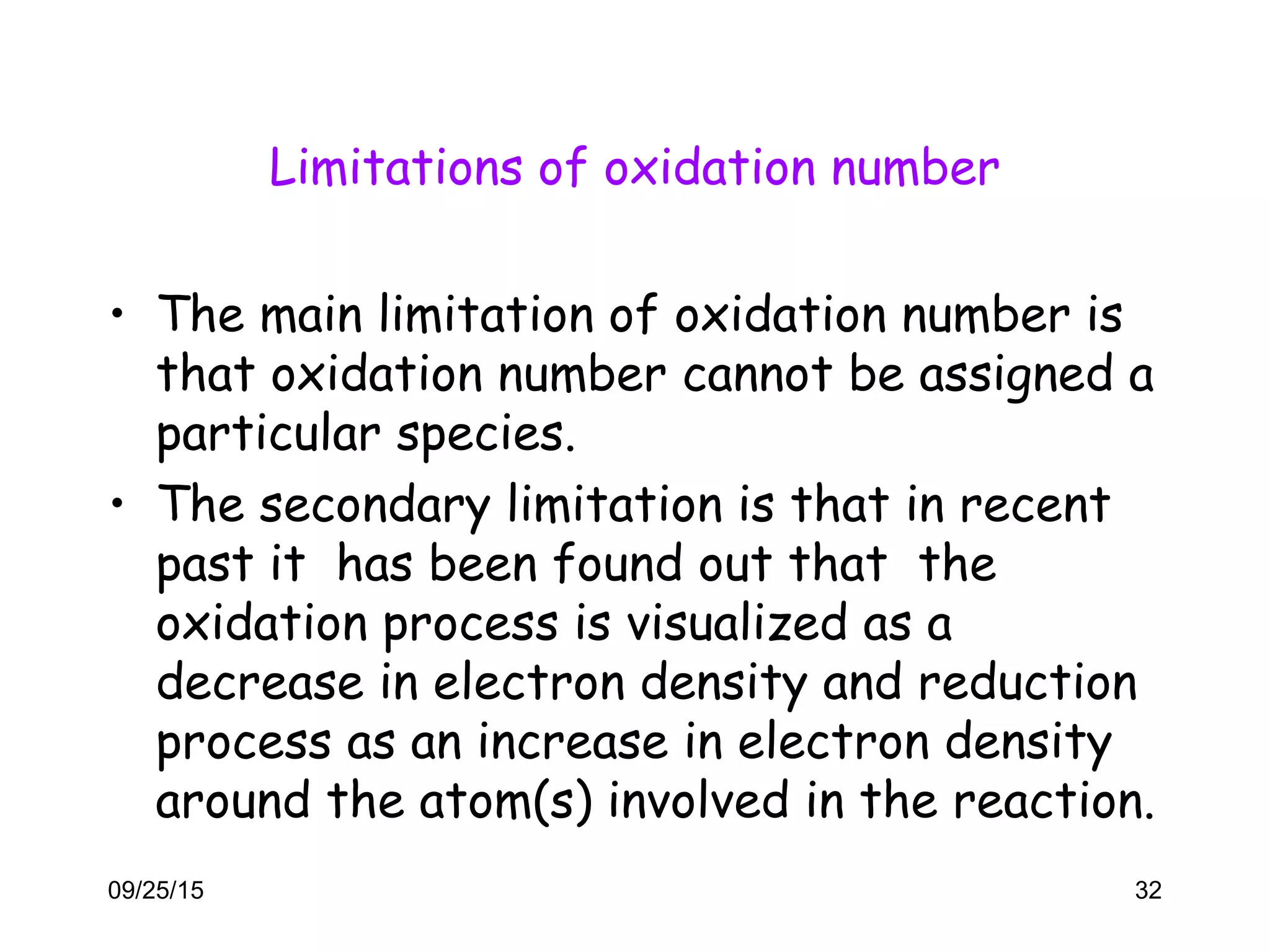
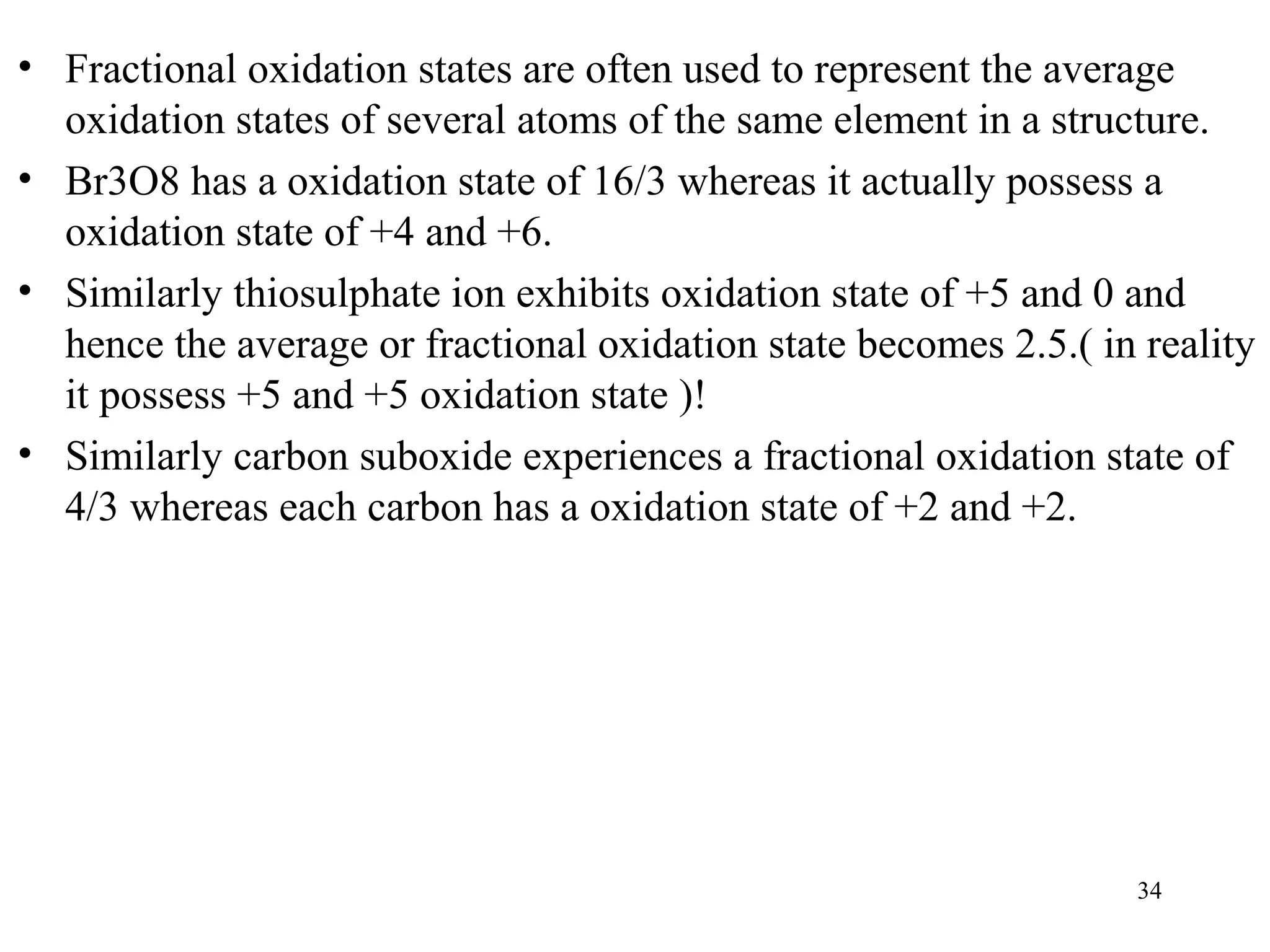
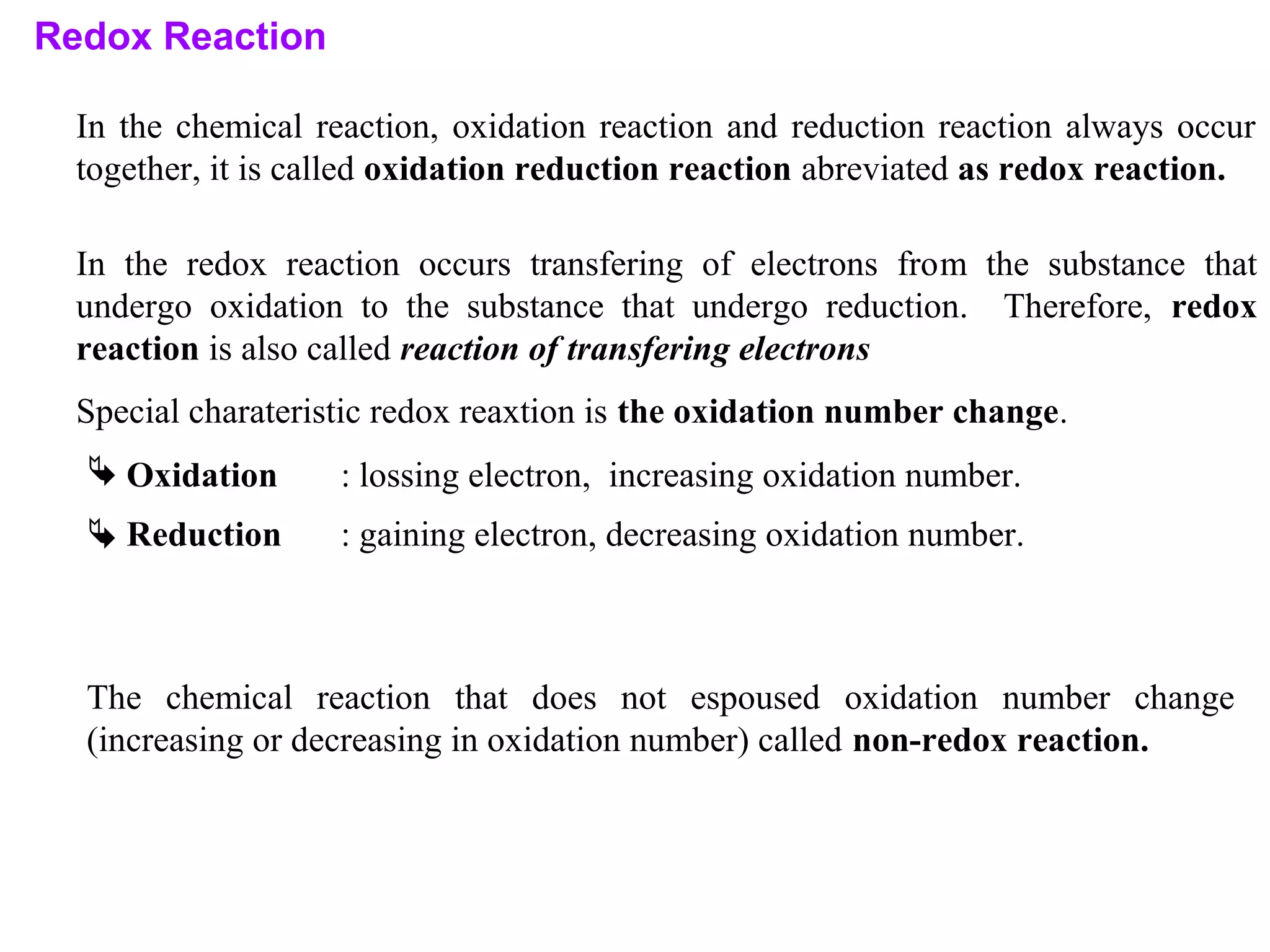
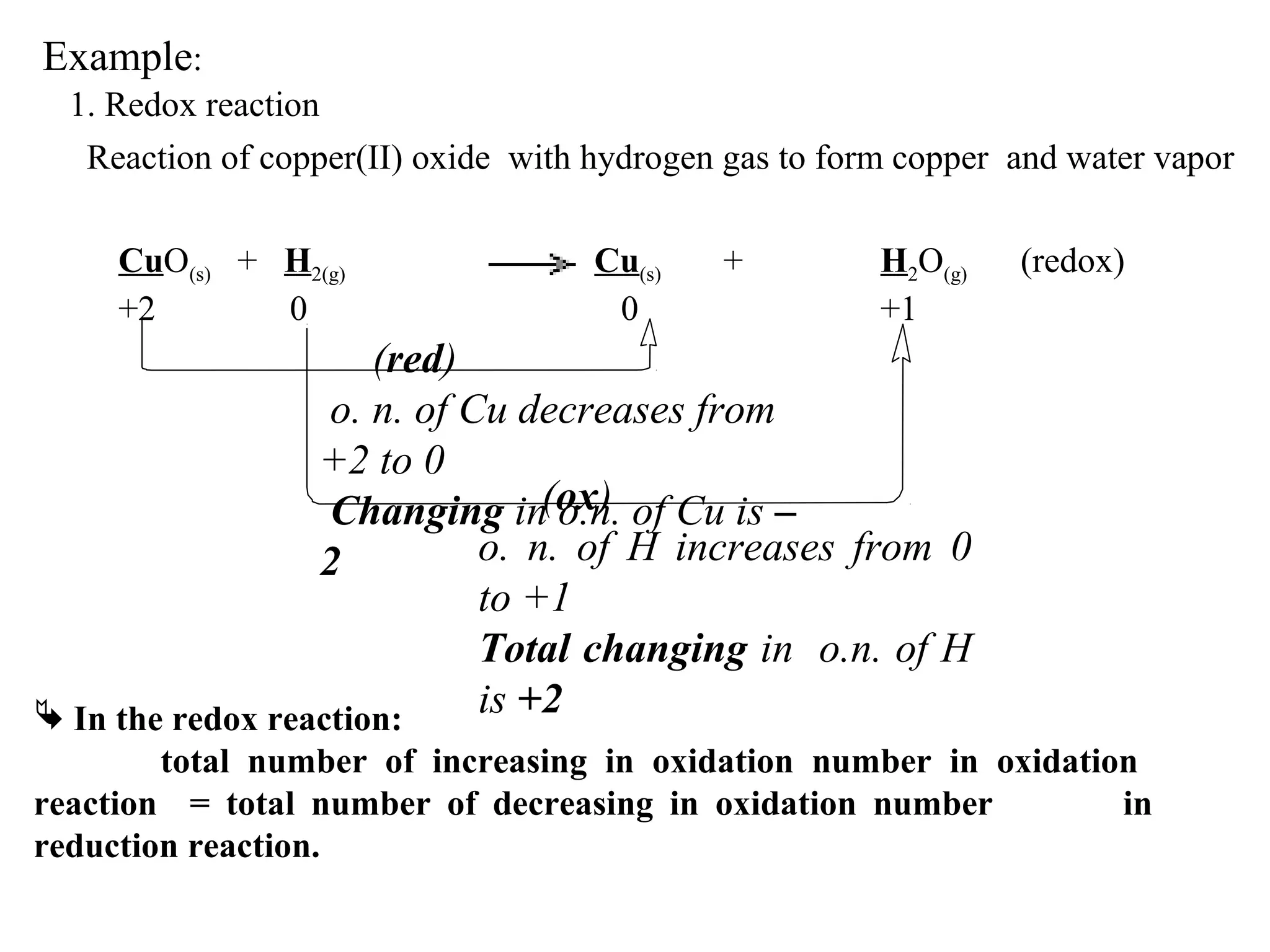
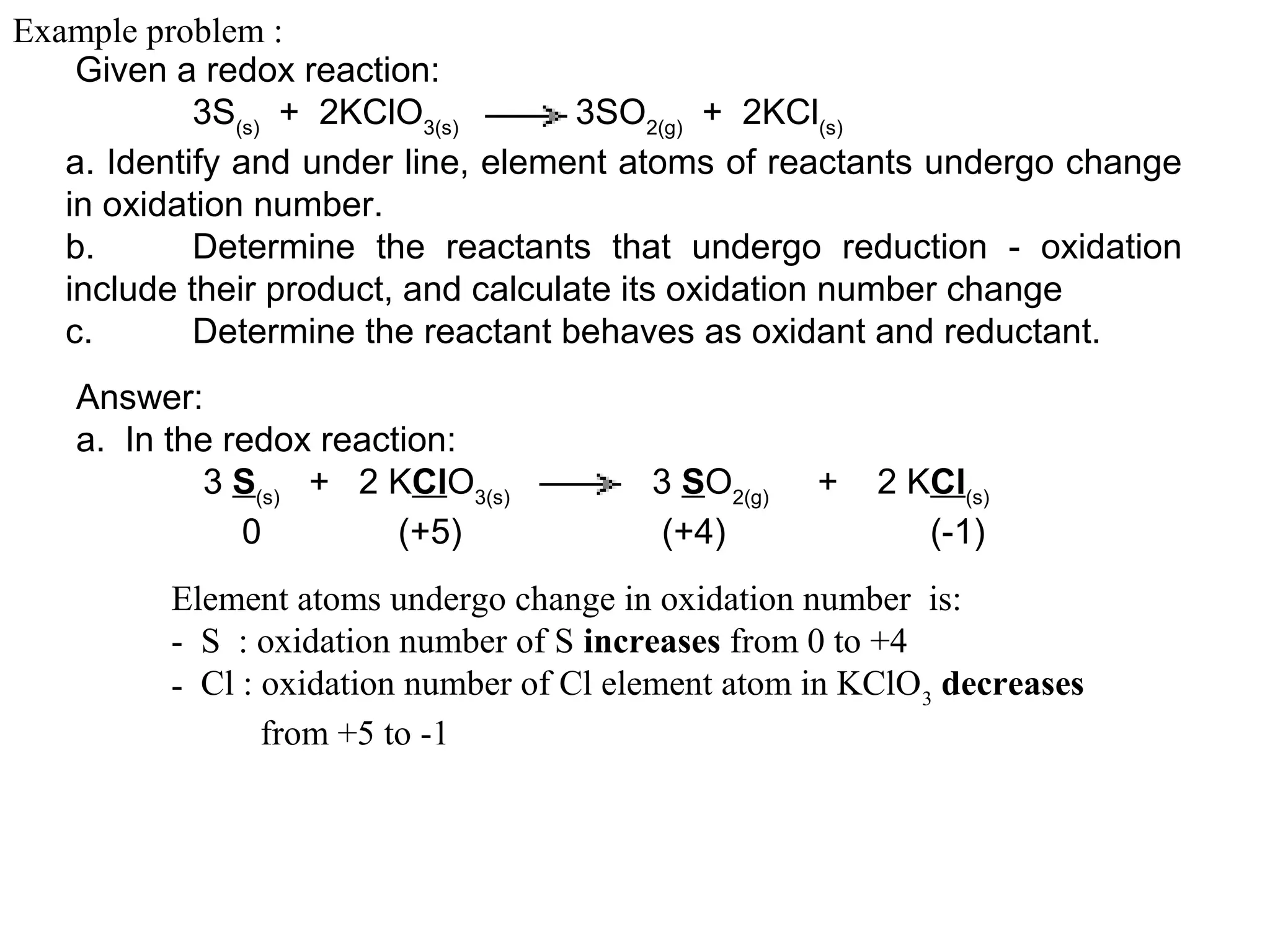
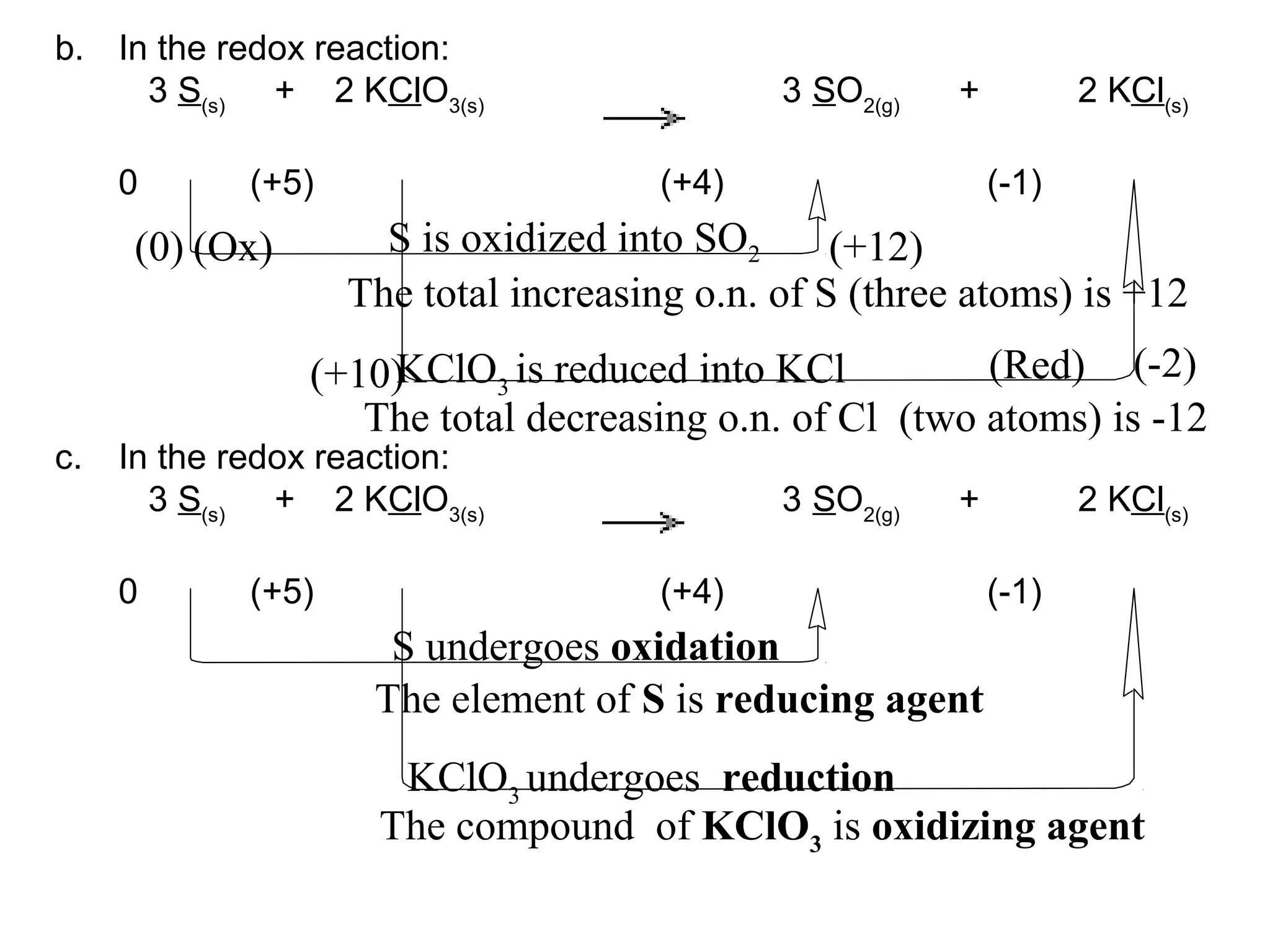
This document discusses oxidation-reduction (redox) reactions and oxidation states. It defines oxidation as the loss of electrons and reduction as the gain of electrons. Redox reactions involve the transfer of electrons from one atom to another. Oxidation numbers are used to track electron transfers and determine if a substance is being oxidized or reduced in a reaction. Common oxidation states of elements are discussed. Rules are provided for determining oxidation numbers based on electronegativity differences in molecules and ions.









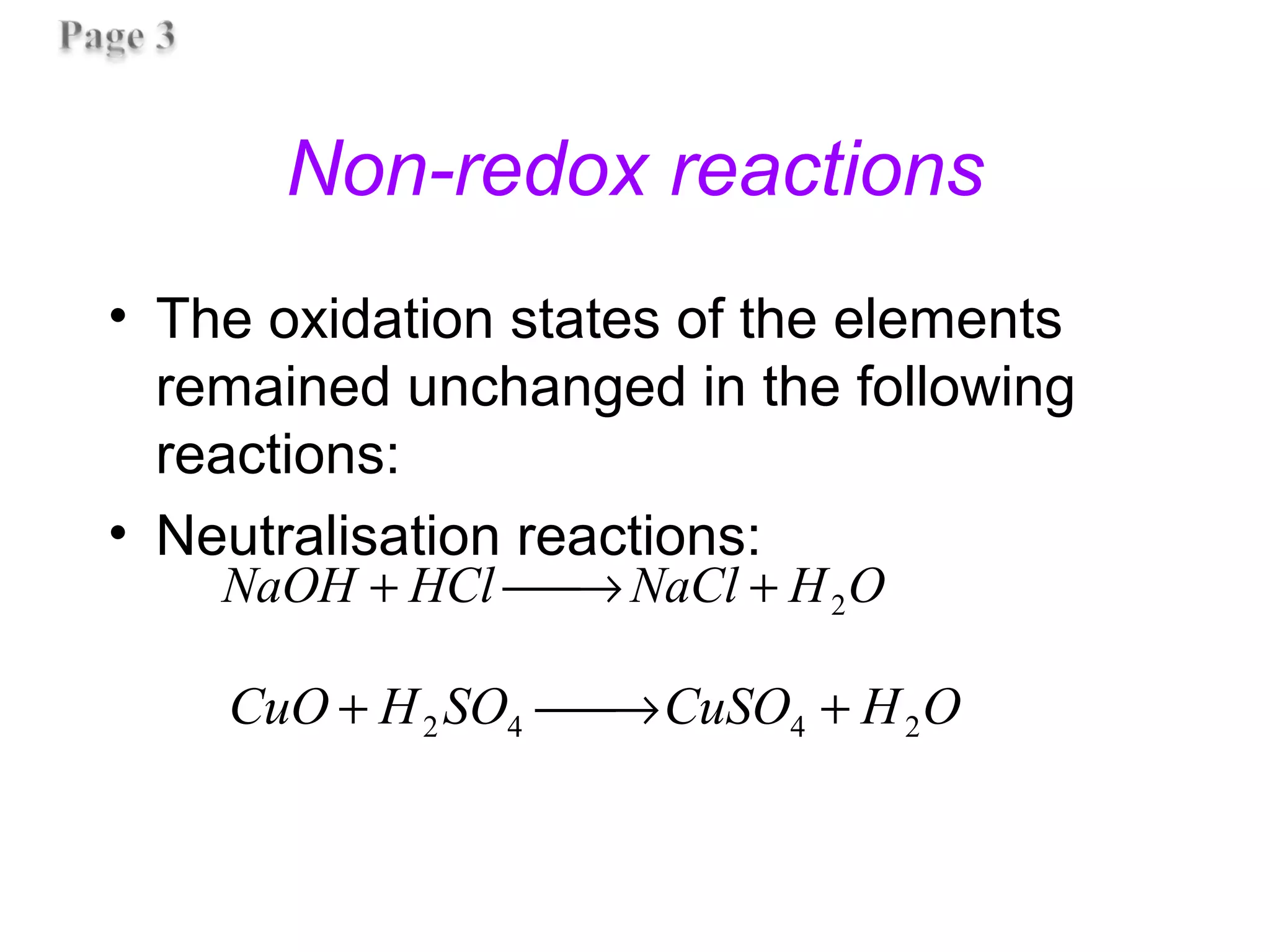
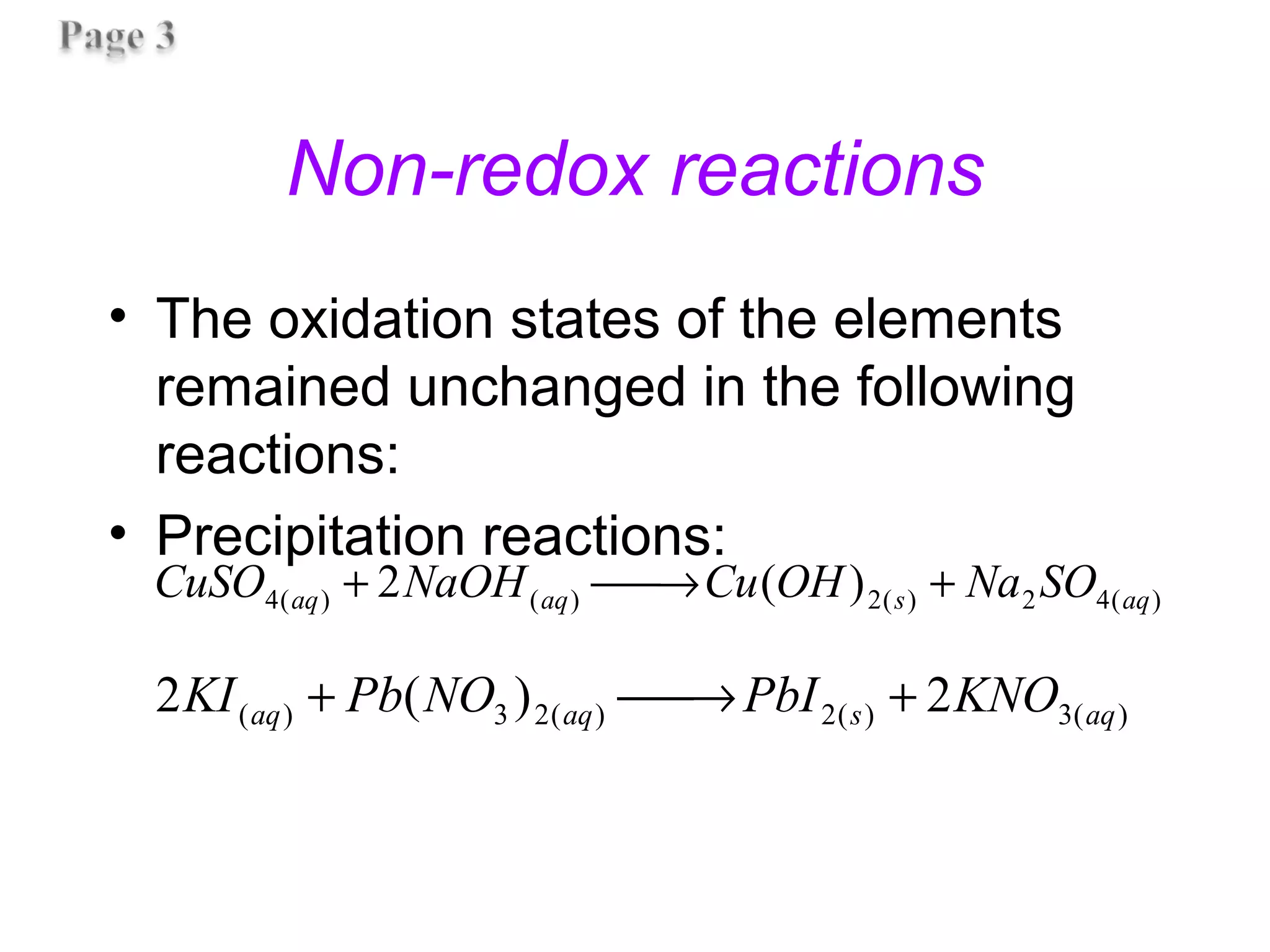
![Non-redox reactions
• The oxidation states of the elements
remained unchanged in the following
reactions:
• Complex formation:
( )[ ] ++
→+
2
)(43)(3)(
2
4 aqaqaq NHCuNHCu
Tetraammine
copper(II) complex
(deep blue solution)
ligand](https://image.slidesharecdn.com/chapter8redoxreactionsppt-150925091407-lva1-app6892/75/Chapter-8-redox-reactions-ppt-for-class-11-CBSE-42-2048.jpg)