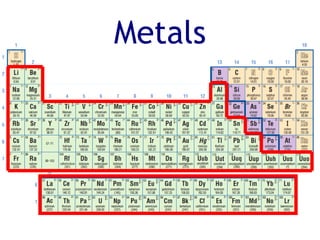
The document summarizes key aspects of the periodic table. It describes how the periodic table is organized into rows called periods and columns called families or groups. Elements in the same group have similar properties. Metals are on the left and center of the table, while non-metals are on the right. Metalloids separate metals and non-metals. Chemical families have elements with similar properties, such as alkali metals in Group 1 reacting easily with water and air. The number of valence electrons increases from left to right across a period and decreases down a group.