Periodic Table of the Elements Lesson PowerPoint
- 1. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 3. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. Copyright © 2010 Ryan P. Murphy
- 4. -Nice neat notes that are legible and use indents when appropriate. -Example of indent. -Skip a line between topics - -Make visuals clear and well drawn. Label please. Neutron Proton Electron
- 5. • RED SLIDE: These are notes that are very important and should be recorded in your science journal. • BLACK SLIDE: Pay attention, follow directions, complete projects as described and answer required questions neatly. Copyright © 2010 Ryan P. Murphy
- 7. • Activity! (Optional) Arranging the Giant Periodic Table of the Elements from last years class. – Try to do without the periodic table. – Bring your periodic table just in case. – You will be timed and compared at the end of the unit. Copyright © 2010 Ryan P. Murphy
- 8. • Activity Sheet Available: Meet the Elements. A Nice Review.
- 9. New Area of Focus: Periodic Table of the Elements. Copyright © 2010 Ryan P. Murphy
- 10. New Area of Focus: Periodic Table of the Elements. Copyright © 2010 Ryan P. Murphy
- 11. • Dimitri Mendeleev, the father of The Periodic Table of the Elements. Copyright © 2010 Ryan P. Murphy
- 12. • Dimitri Mendeleev, the father of The Periodic Table of the Elements. – Made cards of the elements and then began placing them in logical orders. Copyright © 2010 Ryan P. Murphy
- 13. • Dimitri Mendeleev, the father of The Periodic Table of the Elements. – Made cards of the elements and then began placing them in logical orders. – Described elements according to both atomic weight and valence. Copyright © 2010 Ryan P. Murphy
- 14. • Dimitri Mendeleev, the father of The Periodic Table of the Elements. – Made cards of the elements and then began placing them in logical orders. – Described elements according to both atomic weight and valence. Copyright © 2010 Ryan P. Murphy He used his early periodic table to make bold predictions of unknown elements.
- 15. • Dimitri Mendeleev, the father of The Periodic Table of the Elements. – Made cards of the elements and then began placing them in logical orders. – Described elements according to both atomic weight and valence. Copyright © 2010 Ryan P. Murphy He used his early periodic table to make bold predictions of unknown elements. When germanium, gallium and scandium were found they fit perfectly into his periodic table.
- 16. • Dimitri Mendeleev, the father of The Periodic Table of the Elements. – Made cards of the elements and then began placing them in logical orders. – Described elements according to both atomic weight and valence. Copyright © 2010 Ryan P. Murphy He used his early periodic table to make bold predictions of unknown elements. When germanium, gallium and scandium were found they fit perfectly into his periodic table. Biography. Learn more at… http://www.famousscientists.org /dmitri-mendeleev/
- 17. • British chemist Henry Moseley in 1913. Copyright © 2010 Ryan P. Murphy
- 18. • British chemist Henry Moseley in 1913. – He proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. Copyright © 2010 Ryan P. Murphy
- 19. • British chemist Henry Moseley in 1913. – He proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. – This helped reorganize the periodic table. Copyright © 2010 Ryan P. Murphy
- 20. • British chemist Henry Moseley in 1913. – He proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. – This helped reorganize the periodic table. Copyright © 2010 Ryan P. Murphy Enlisted with the British Army and was killed August 1914, by sniper in World War I.
- 21. • British chemist Henry Moseley in 1913. – He proposed that the atom contains in its nucleus a number of positive nuclear charges that is equal to its (atomic) number in the periodic table. – This helped reorganize the periodic table. Copyright © 2010 Ryan P. Murphy Enlisted with the British Army and was killed August 1914, by sniper in World War I. Learn more at…… http://www.famousscientists.org/henry-moseley/
- 22. • Activity! – Your table group is going to get a group of cards. Copyright © 2010 Ryan P. Murphy
- 23. • Activity! – Your table group is going to get a group of cards. – Each table one at a time will lay down the cards in a logical order. Copyright © 2010 Ryan P. Murphy
- 24. • Activity! – Your table group is going to get a group of cards. – Each table one at a time will lay down the cards in a logical order. Think Dimitri Mendeleev and organizing according to valence and atomic mass. Copyright © 2010 Ryan P. Murphy
- 25. Copyright © 2010 Ryan P. Murphy
- 26. Copyright © 2010 Ryan P. Murphy
- 27. Copyright © 2010 Ryan P. Murphy
- 28. Copyright © 2010 Ryan P. Murphy
- 29. Copyright © 2010 Ryan P. Murphy
- 30. Copyright © 2010 Ryan P. Murphy
- 31. Copyright © 2010 Ryan P. Murphy
- 32. Copyright © 2010 Ryan P. Murphy
- 33. Copyright © 2010 Ryan P. Murphy
- 34. • Questions – Which were missing? How do you know? – How is the periodic table similar to the arrangements of cards? Copyright © 2010 Ryan P. Murphy
- 35. • Questions – Which were missing? How do you know? Copyright © 2010 Ryan P. Murphy
- 36. • Questions – Which were missing? How do you know? – 5, J, 2, 6, 7, 7, J, 3 Copyright © 2010 Ryan P. Murphy
- 37. • Questions – How is the periodic table similar to the arrangements of cards? Copyright © 2010 Ryan P. Murphy
- 38. • Answer! Copyright © 2010 Ryan P. Murphy
- 39. • Answer! – The Periodic Table increases in amu from left to right. Copyright © 2010 Ryan P. Murphy
- 40. • Answer! – The Periodic Table increases in amu from left to right. Copyright © 2010 Ryan P. Murphy
- 41. • Answer! – The Periodic Table increases in amu from left to right. – Groups show the same number of valence E- Copyright © 2010 Ryan P. Murphy
- 42. • Answer! – The Periodic Table increases in amu from left to right. – Groups show the same number of valence E- Copyright © 2010 Ryan P. Murphy
- 43. • Who are these two scientists and what did they do? Copyright © 2010 Ryan P. Murphy
- 44. • Who are these two scientists and what did they do? Copyright © 2010 Ryan P. Murphy
- 45. • Who are these two scientists and what did they do? Copyright © 2010 Ryan P. Murphy Henry Moseley helped reorganize the periodic table according to atomic number.
- 46. • Who are these two scientists and what did they do? Copyright © 2010 Ryan P. Murphy Henry Moseley helped reorganize the periodic table according to atomic number.
- 47. • Who are these two scientists and what did they do? Copyright © 2010 Ryan P. Murphy Dimitri Mendeleev, the father of The Periodic Table of the Elements. Henry Moseley helped reorganize the periodic table according to atomic number.
- 48. The Periodic Table of the Elements is a… - - - - Copyright © 2010 Ryan P. Murphy
- 49. A chart of all the known elements. Copyright © 2010 Ryan P. Murphy
- 50. Is in order of increasing atomic number and mass. Copyright © 2010 Ryan P. Murphy
- 51. Is in order of increasing atomic number and mass. Copyright © 2010 Ryan P. Murphy
- 52. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Atomic Mass and Atomic Number increases as you move across and down. Copyright © 2010 Ryan P. Murphy
- 53. The table puts elements into groups with similar characteristics. Copyright © 2010 Ryan P. Murphy
- 54. The table puts elements into groups with similar characteristics. Copyright © 2010 Ryan P. Murphy
- 55. Allows us to recognize trends over the whole array of elements. Copyright © 2010 Ryan P. Murphy
- 56. • The periodic table can also be used as a way to write love letters in class.
- 57. • The periodic table can also be used as a way to write love letters in class.
- 58. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 59. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 60. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 61. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy One orbital
- 62. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy One orbital Valence Electrons
- 63. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 64. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 65. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy Two Orbitals
- 66. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 67. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 68. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy Three Orbitals
- 69. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 70. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 71. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy Four Orbitals
- 72. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 73. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 74. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy Five Orbitals
- 75. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 76. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 77. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy Six Orbital
- 78. • All of the elements in a period have the same number of atomic orbitals. Copyright © 2010 Ryan P. Murphy
- 84. • – It is grouped with the alkali metals because it has a similar outer shell electron configuration as they do. Copyright © 2010 Ryan P. Murphy
- 85. • Hydrogen is an odd ball. – It is grouped with the alkali metals because it has a similar outer shell electron configuration as they do. Copyright © 2010 Ryan P. Murphy
- 86. • Hydrogen is an odd ball. Copyright © 2010 Ryan P. Murphy
- 87. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. Copyright © 2010 Ryan P. Murphy
- 88. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Copyright © 2010 Ryan P. Murphy
- 89. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Copyright © 2010 Ryan P. Murphy
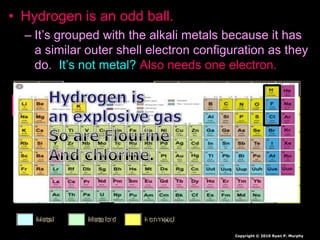
- 90. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 91. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 92. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 93. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 94. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 95. Copyright © 2010 Ryan P. Murphy
- 96. • How are Nitrogen and Phosphorus similar? Copyright © 2010 Ryan P. Murphy
- 97. • How are Nitrogen and Phosphorus similar? – They both have 5 electrons in their outermost shell. Copyright © 2010 Ryan P. Murphy
- 98. Copyright © 2010 Ryan P. Murphy
- 99. • How are Boron and Gallium similar? Copyright © 2010 Ryan P. Murphy
- 100. • How are Boron and Gallium similar? – They both have 3 electrons in their outermost shell. Copyright © 2010 Ryan P. Murphy
- 101. • How are Boron and Gallium similar? – They both have 3 electrons in their outermost shell. – The Boron Family Group (13 group) have ns2np1 in their outer shell Copyright © 2010 Ryan P. Murphy
- 102. • How are Boron and Gallium similar? – They both have 3 electrons in their outermost shell. – The Boron Family Group (13 group) have ns2np1 in their outer shell Copyright © 2010 Ryan P. Murphy I prefer the standard Periodic Table, however, new periodic tables have found creative ways to arrange the elements.
- 103. Copyright © 2010 Ryan P. Murphy
- 105. • Quiz! – Memorize the first 10 elements and their order from 1-10 in 7 minutes on The Periodic Table of Elements. Copyright © 2010 Ryan P. Murphy
- 106. • Video Song to help memorize the first ten elements. – http://www.youtube.com/watch?v=OqtgPcAS GVI
- 107. • Please say the remaining 100 elements in 1 minute and 25 seconds. – Less than Tom Lehrers. – You get to use your table…1 minute to practice and your time starts now! Copyright © 2010 Ryan P. Murphy
- 108. • Video song! Tom Lehrers (1:25 seconds) • http://www.youtube.com/watch?v=DYW50 F42ss8
- 109. • Video song! Tom Lehrers • http://www.youtube.com/watch?v=nHUo 0lG8Gi0
- 110. • Interactive Periodic Table of the Elements • http://www.ptable.com/
- 111. Horizontal row is called Period Copyright © 2010 Ryan P. Murphy
- 112. Horizontal row is called Period (Same # of electron orbitals) Copyright © 2010 Ryan P. Murphy
- 113. Horizontal row is called Period (Same # of electron orbitals) Vertical column is called group/family. Copyright © 2010 Ryan P. Murphy
- 114. Horizontal row is called Period (Same # of electron orbitals) Vertical column is called group/family. (Same # of valence electrons) Copyright © 2010 Ryan P. Murphy
- 115. • Is the circled area a period or group on the periodic table? Copyright © 2010 Ryan P. Murphy
- 116. • Is the circled area a period or group on the periodic table? Answer: Group Copyright © 2010 Ryan P. Murphy
- 117. • Is the circled area a period or group on the periodic table? Answer: Group Copyright © 2010 Ryan P. Murphy
- 118. • Is the circled area a period or group on the periodic table? Answer: Group Copyright © 2010 Ryan P. Murphy
- 119. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr G R O U P Copyright © 2010 Ryan P. Murphy
- 120. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr PERIOD
- 121. AMU increases from left to right and top to bottom. Copyright © 2010 Ryan P. Murphy
- 122. AMU increases from left to right and top to bottom. Copyright © 2010 Ryan P. Murphy
- 123. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr AMU increases as you go from left to right, and from top to bottom Copyright © 2010 Ryan P. Murphy
- 124. Electronegativity increases from lower left to upper right. Copyright © 2010 Ryan P. Murphy
- 125. Electronegativity increases from lower left to upper right. Copyright © 2010 Ryan P. Murphy Moving top to bottom down the periodic table, electronegativity decreases.
- 126. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Copyright © 2010 Ryan P. Murphy
- 127. Copyright © 2010 Ryan P. Murphy Note: Noble gases are missing.
- 128. Copyright © 2010 Ryan P. Murphy
- 129. • The most strongly electronegative element, Fluorine (F). Copyright © 2010 Ryan P. Murphy
- 130. • The most strongly electronegative element, Fluorine (F). Copyright © 2010 Ryan P. Murphy “I want electrons.”
- 131. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy
- 132. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away one electron.”
- 133. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away one electron.” “I want to gain one electron”
- 134. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away one electron.” “I want to gain one electron”
- 135. • The most strongly electronegative element, Fluorine (F). • The least electronegative element is Francium (Fr). Copyright © 2010 Ryan P. Murphy “I want to give away one electron.” “I want to gain one electron” “You guys should get together.”
- 136. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. Copyright © 2010 Ryan P. Murphy
- 137. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy
- 138. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy “Those elements attract electrons like wicked.”
- 139. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy “Not the Noble Gases however.”
- 140. • Electronegativity is a measure of the attraction of an atom for the electrons in a chemical bond. – The higher the electronegativity of an atom, the greater its attraction for bonding electrons. Copyright © 2010 Ryan P. Murphy “Not the Noble Gases however.” “They’re wicked different.”
- 141. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 142. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 143. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 144. – Electrons with low ionization energies have a low electronegativity because their nuclei do not exert a strong attractive force on electrons. – Elements with high ionization energies have a high electronegativity due to the strong pull exerted on electrons by the nucleus. Copyright © 2010 Ryan P. Murphy and Ions)Ionization energy is the energy required to remove an electron. (Gases and Ions)
- 145. Transition Metals are found in the middle. Copyright © 2010 Ryan P. Murphy
- 149. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Transition Metals Copyright © 2010 Ryan P. Murphy
- 150. Transition Metals are… - - - - - Copyright © 2010 Ryan P. Murphy
- 151. Malleable: To be shaped / made into sheets. Copyright © 2010 Ryan P. Murphy
- 153. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Wrap coin in foil limiting creases a press from above onto foil to make imprint. – Cut foil around quarter using scissors. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency. Indium used here instead of aluminum foil
- 154. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Wrap coin in foil limiting creases a press from above onto foil to make imprint. – Cut foil around quarter using scissors. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency. Indium used here instead of aluminum foil
- 155. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Make many imprints of he coin in the very malleable aluminum foil. • Can use journal to press the foil around coins. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency.
- 156. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Make many imprints of he coin in the very malleable aluminum foil. • Can use journal to press the foil around coins. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency.
- 157. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Make many imprints of he coin in the very malleable aluminum foil. • Can use journal to press the foil around coins. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency.
- 158. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Make many imprints of he coin in the very malleable aluminum foil. • Can use journal to press the foil around coins. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency.
- 159. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Make many imprints of he coin in the very malleable aluminum foil. • Can use journal to press the foil around coins. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency.
- 160. • Activity! Counterfeiting Coins. – Not really, but don’t tell the feds about today. – Everyone is loaned one quarter and given a small piece of heavy duty aluminum foil. – Make many imprints of he coin in the very malleable aluminum foil. • Can use journal to press the foil around coins. – Hand quarter back to teacher and don’t use quarter imprint as any form of currency.
- 161. Ductile: Made into wire.
- 166. • Video Link! Picking a lock with a paperclip. – (Very Optional) For future lock smiths out there. – https://www.youtube.com/watch?v=rZTtuXkrXjc
- 167. • Video Link! Attaching two paperclips together with a dollar bill. – Teacher loans the bills and paperclips. – Watch video and perform in real time. • https://www.youtube.com/watch?v=vic6CjUv32M
- 168. • Video Link! Attaching two paperclips together with a dollar bill. – Teacher loans the bills and paperclips. – Watch video and perform in real time. • https://www.youtube.com/watch?v=vic6CjUv32M
- 169. • Video Link! Attaching two paperclips together with a dollar bill. – Teacher loans the bills and paperclips. – Watch video and perform in real time. • https://www.youtube.com/watch?v=vic6CjUv32M
- 170. • Video Link! Attaching two paperclips together with a dollar bill. – Teacher loans the bills and paperclips. – Watch video and perform in real time. • https://www.youtube.com/watch?v=vic6CjUv32M
- 171. Good conductors of electricity. Copyright © 2010 Ryan P. Murphy
- 172. • Copper (Cu) is a good conductor of electricity. – It is malleable and ductile. Copyright © 2010 Ryan P. Murphy
- 173. • Activity! Find something that is a good conductor of electricity. – Test with the conductivity meter. Copyright © 2010 Ryan P. Murphy
- 174. Have a high luster (shine). Copyright © 2010 Ryan P. Murphy
- 175. Have a high luster (shine). Copyright © 2010 Ryan P. Murphy
- 176. Have a high luster (shine). Copyright © 2010 Ryan P. Murphy
- 177. Conducts heat well. Copyright © 2010 Ryan P. Murphy
- 178. Conducts heat well. Copyright © 2010 Ryan P. Murphy
- 179. Conducts heat well. Copyright © 2010 Ryan P. Murphy
- 180. Most have a high density. Copyright © 2010 Ryan P. Murphy
- 181. Most have a high density. Copyright © 2010 Ryan P. Murphy
- 182. Most have a high density. Copyright © 2010 Ryan P. Murphy
- 183. Most have a high density. Copyright © 2010 Ryan P. Murphy
- 184. Most have a high density. Copyright © 2010 Ryan P. Murphy
- 185. Most are solid. Hg (mercury is a liquid metal) Copyright © 2010 Ryan P. Murphy
- 186. Most are solid. Hg (mercury is a liquid metal) Copyright © 2010 Ryan P. Murphy
- 187. Most are solid. Hg (mercury is a liquid metal) Copyright © 2010 Ryan P. Murphy
- 188. Most are solid. Hg (mercury is a liquid metal) Copyright © 2010 Ryan P. Murphy
- 189. • Field Trip! Let’s check out some mercury and see why it is used the way it is? Copyright © 2010 Ryan P. Murphy
- 190. • Thermostats with Mercury: – Since mercury is a liquid it travels downhill. – When the dial is turned on, the mercury travels down and connects wires telling the heater to turn on. – When thermostat is turned off, the connection is broken. Copyright © 2010 Ryan P. Murphy
- 191. Metallically bonded. Copyright © 2010 Ryan P. Murphy
- 192. Metallically bonded. Copyright © 2010 Ryan P. Murphy
- 193. Metallically bonded. Copyright © 2010 Ryan P. Murphy
- 194. Many metals are reactive to chemicals. Copyright © 2010 Ryan P. Murphy
- 195. Many metals are reactive to chemicals. Copyright © 2010 Ryan P. Murphy
- 196. Many metals are reactive to chemicals. Copyright © 2010 Ryan P. Murphy
- 197. Almost 75% of all elements are classified as metals. Copyright © 2010 Ryan P. Murphy
- 198. Almost 75% of all elements are classified as metals. Copyright © 2010 Ryan P. Murphy
- 199. Almost 75% of all elements are classified as metals. Copyright © 2010 Ryan P. Murphy
- 200. Alloys: Metals are easily combined Copyright © 2010 Ryan P. Murphy
- 201. • Bronze age: Copper and tin Copyright © 2010 Ryan P. Murphy
- 202. • Continued Metals… Copyright © 2010 Ryan P. Murphy
- 203. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 204. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 205. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 206. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 207. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 208. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 209. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 210. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 211. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 212. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 213. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 214. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 215. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 216. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 217. • Some of the metals. Use your table…. – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals Copyright © 2010 Ryan P. Murphy
- 218. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 219. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 220. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 221. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals http://www.youtube.com/watch?v=QiQoMDZGCs4
- 222. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 223. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 224. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 225. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 226. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 227. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, Rare Metals, Rare-Earth Metals, and Transition Metals
- 228. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, and Transition Metals.
- 229. • Some of the metals – Actinide Metals, Lanthanide Metals, Alkali Metals, Alkaline-Earth Metals, Noble Metals, and Transition Metals.
- 230. • Demonstration! Copyright © 2010 Ryan P. Murphy
- 231. • Demonstration! – Thermite Reaction Copyright © 2010 Ryan P. Murphy
- 232. • Demonstration! – Thermite Reaction – The Aluminum reduces the oxide of another metal, most commonly iron oxide, because aluminum is highly combustible: Copyright © 2010 Ryan P. Murphy
- 233. • Demonstration! – Thermite Reaction – The Aluminum reduces the oxide of another metal, most commonly iron oxide, because aluminum is highly combustible: • Fe2O3 + 2Al → 2 Fe + Al2O3 + heat Copyright © 2010 Ryan P. Murphy
- 234. • Demonstration! – Thermite Reaction – The Aluminum reduces the oxide of another metal, most commonly iron oxide, because aluminum is highly combustible: • Fe2O3 + 2Al → 2 Fe + Al2O3 + heat Copyright © 2010 Ryan P. Murphy
- 235. • Demonstration! – Thermite Reaction – The Aluminum reduces the oxide of another metal, most commonly iron oxide, because aluminum is highly combustible: • Fe2O3 + 2Al → 2 Fe + Al2O3 + heat Copyright © 2010 Ryan P. Murphy
- 236. • Demonstration! – Thermite Reaction – The Aluminum reduces the oxide of another metal, most commonly iron oxide, because aluminum is highly combustible: • Fe2O3 + 2Al → 2 Fe + Al2O3 + heat • http://www.youtube.com/watch?v=O5v3XxFfUOw &feature=related Copyright © 2010 Ryan P. Murphy
- 237. • 1st Group Alkali Metals (Orange) Copyright © 2010 Ryan P. Murphy
- 238. • 1st Group Alkali Metals (Orange) – One valence electron Copyright © 2010 Ryan P. Murphy
- 239. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Alkali Metals Copyright © 2010 Ryan P. Murphy
- 241. • Alkali metals have one valence electron
- 242. • Alkali metals have one valence electron – Sodium
- 243. • Alkali metals have one valence electron • Halogens have seven valence electrons – Sodium
- 244. • Alkali metals have one valence electron • Halogens have seven valence electrons – Sodium - Chlorine
- 249. Sodium Chloride
- 250. Sodium Chloride Sodium has an electronegativity of 1.0 Chlorine has an electronegativity of 3.0
- 251. Sodium Chloride Sodium has an electronegativity of 1.0 Chlorine has an electronegativity of 3.0 3.0 –1.0 =
- 252. Sodium Chloride Sodium has an electronegativity of 1.0 Chlorine has an electronegativity of 3.0 3.0 –1.0 = 2 Electron diff.
- 253. Sodium Chloride Sodium has an electronegativity of 1.0 Chlorine has an electronegativity of 3.0 3.0 –1.0 = 2 Electron diff. Electronegativity Difference Type of Bond Formed 0.0 to 0.2 nonpolar covalent 0.3 to 1.4 polar covalent > 1.5 ionic
- 254. Sodium Chloride Sodium has an electronegativity of 1.0 Chlorine has an electronegativity of 3.0 3.0 –1.0 = 2 Electron diff. Electronegativity Difference Type of Bond Formed 0.0 to 0.2 nonpolar covalent 0.3 to 1.4 polar covalent > 1.5 ionic
- 255. Sodium Chloride Sodium has an electronegativity of 1.0 Chlorine has an electronegativity of 3.0 3.0 –1.0 = 2 Electron diff. Electronegativity Difference Type of Bond Formed 0.0 to 0.2 nonpolar covalent 0.3 to 1.4 polar covalent > 1.5 ionic Very Polar
- 256. • Video: Alkali Metals and water. – Apologies for the moderately inappropriate expression that is used. – http://www.youtube.com/watch?v=m55kgyApYrY Copyright © 2010 Ryan P. Murphy
- 257. • Francium
- 258. • Francium
- 259. • Francium: Incredibly reactive in water.
- 260. • Francium: Incredibly reactive in water. Hardly any Francium occurs naturally in the earth's crust.
- 266. “I’m really far from the nucleus.
- 267. “I’m really far from the nucleus. It takes less energy to remove that outer electron from the atom.
- 268. “I’m really far from the nucleus. It takes less energy to remove that outer electron from the atom. This atom has a very low ionization energy.
- 269. “I’m really far from the nucleus. It takes less energy to remove that outer electron from the atom. This atom has a very low ionization energy. Also the lowest electronegativity of any element.
- 270. • The Alkaline Earth Elements are metallic elements found in the second period of the periodic table Copyright © 2010 Ryan P. Murphy
- 271. • The Alkaline Earth Elements are metallic elements found in the second period of the periodic table (Aqua). Copyright © 2010 Ryan P. Murphy
- 272. • The Alkaline Earth Elements are metallic elements found in the second period of the periodic table (Aqua). – Two valence electrons Copyright © 2010 Ryan P. Murphy
- 273. • The Alkaline Earth Elements are metallic elements found in the second period of the periodic table (Aqua). – Two valence electrons Copyright © 2010 Ryan P. Murphy
- 274. • The Alkaline Earth Elements are metallic elements found in the second period of the periodic table (Aqua). – Two valence electrons Copyright © 2010 Ryan P. Murphy
- 275. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Alkaline Earth Metals Copyright © 2010 Ryan P. Murphy
- 276. • What Alkaline Earth metal is this?
- 277. • Answer! Calcium Atomic # 20
- 278. • Flame test – Can be used to visually determine the identity of an unknown metal or metalloid ion based on the characteristic color. – The heat of the flame converts the metal ions into atoms which become excited and emit visible light. – The characteristic emission spectra can be used to differentiate between some elements. • Learn more at… • http://www.chem.purdue.edu/bcce/Scaling_a_flashy _demonstration.pdf
- 279. • Flame test – Can be used to visually determine the identity of an unknown metal or metalloid ion based on the characteristic color when burned. – The heat of the flame converts the metal ions into atoms which become excited and emit visible light. – The characteristic emission spectra can be used to differentiate between some elements. • Learn more at… • http://www.chem.purdue.edu/bcce/Scaling_a_flashy _demonstration.pdf
- 280. • Flame test – Can be used to visually determine the identity of an unknown metal or metalloid ion based on the characteristic color when burned. – The heat of the flame converts the metal ions into atoms which become excited and emit visible light. – The characteristic emission spectra can be used to differentiate between some elements. • Learn more at… • http://www.chem.purdue.edu/bcce/Scaling_a_flashy _demonstration.pdf
- 281. • Flame test – Can be used to visually determine the identity of an unknown metal or metalloid ion based on the characteristic color when burned. – The heat of the flame converts the metal ions into atoms which become excited and emit visible light. – The characteristic emission spectra can be used to differentiate between some elements.
- 282. • Video Link Flame Test – https://www.youtube.com/watch?v=jJvS4uc4TbU
- 283. • How it works again. – When the atoms of a gas or vapor are excited (Heat), their electrons are able to move from their ground state to higher energy levels.
- 284. • How it works again. – When the atoms of a gas or vapor are excited (Heat), their electrons are able to move from their ground state to higher energy levels. – As go back to their ground state, they emit photons of very specific energy.
- 285. • How it works again. – When the atoms of a gas or vapor are excited (Heat), their electrons are able to move from their ground state to higher energy levels. – As go back to their ground state, they emit photons of very specific energy. • This energy corresponds to particular wavelengths of light.
- 286. • How it works again. – When the atoms of a gas or vapor are excited (Heat), their electrons are able to move from their ground state to higher energy levels. – As go back to their ground state, they emit photons of very specific energy. • This energy corresponds to particular wavelengths of light.
- 287. • How it works again. – When the atoms of a gas or vapor are excited (Heat), their electrons are able to move from their ground state to higher energy levels. – As go back to their ground state, they emit photons of very specific energy. • This energy corresponds to particular wavelengths of light. Learn more / conduct demonstration at… http://www.creative-chemistry.org.uk/activities/flametests.htm http://www.chem.purdue.edu/bcce/Scaling_a_flashy_demonstration.pdf
- 288. Metalloids / Semi metals: Properties of metals and non-metals - - - Copyright © 2010 Ryan P. Murphy
- 289. Semi-conductors Copyright © 2010 Ryan P. Murphy
- 290. • What is this?
- 291. • Answer: Peanut Brittle – What’s the point here?
- 292. • Answer: Peanut Brittle – What’s the point here? – It’s brittle, and so are some of the metalloids.
- 293. Brittle Copyright © 2010 Ryan P. Murphy
- 294. Brittle Copyright © 2010 Ryan P. Murphy
- 295. Brittle Copyright © 2010 Ryan P. Murphy
- 296. Can have luster. Copyright © 2010 Ryan P. Murphy
- 297. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Metalloids / Semimetals Copyright © 2010 Ryan P. Murphy
- 298. Non-Metals Not metals Copyright © 2010 Ryan P. Murphy
- 299. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Non-metals Copyright © 2010 Ryan P. Murphy H
- 300. Non-metals… - - - - - Copyright © 2010 Ryan P. Murphy
- 301. H and He are non-metals. Copyright © 2010 Ryan P. Murphy
- 302. H and He are non-metals. Copyright © 2010 Ryan P. Murphy Remember, Hydrogen is an oddball.
- 303. They are poor conductors. Copyright © 2010 Ryan P. Murphy
- 304. They are poor conductors. Copyright © 2010 Ryan P. Murphy
- 305. They are poor conductors. Copyright © 2010 Ryan P. Murphy
- 306. They are poor conductors. Copyright © 2010 Ryan P. Murphy
- 307. They are poor conductors. Copyright © 2010 Ryan P. Murphy
- 308. They are poor conductors. Copyright © 2010 Ryan P. Murphy
- 309. They are brittle (break when hit). Copyright © 2010 Ryan P. Murphy
- 310. They are brittle (break when hit). Copyright © 2010 Ryan P. Murphy
- 311. They are brittle (break when hit). Copyright © 2010 Ryan P. Murphy
- 312. Dull in color. (No Luster) Copyright © 2010 Ryan P. Murphy
- 313. Dull in color. (No Luster) Copyright © 2010 Ryan P. Murphy
- 314. Dull in color. (No Luster) Copyright © 2010 Ryan P. Murphy
- 315. Poor conductors of heat. Copyright © 2010 Ryan P. Murphy
- 316. Poor conductors of heat. Copyright © 2010 Ryan P. Murphy
- 317. Poor conductors of heat. Copyright © 2010 Ryan P. Murphy
- 318. They may be transparent or translucent. Copyright © 2010 Ryan P. Murphy
- 319. They may be transparent or translucent. Copyright © 2010 Ryan P. Murphy
- 320. They exist as a… Copyright © 2010 Ryan P. Murphy
- 321. They exist as a… (s), Copyright © 2010 Ryan P. Murphy Solid
- 322. They exist as a… (s), (l), Copyright © 2010 Ryan P. Murphy Solid Liquid
- 323. They exist as a… (s), (l), (g). Copyright © 2010 Ryan P. Murphy Solid Liquid Gas
- 324. They exist as a… (s), (l), (g). Copyright © 2010 Ryan P. Murphy Solid Liquid Gas S Sulfur
- 325. They exist as a… (s), (l), (g). Copyright © 2010 Ryan P. Murphy Solid Liquid Gas S Sulfur Br Bromine
- 326. They exist as a… (s), (l), (g). Copyright © 2010 Ryan P. Murphy Solid Liquid Gas S Sulfur Br Bromine Cl Chlorine
- 327. • Is this extremely dangerous to use in your home?
- 328. • Is this extremely dangerous to use in your home? • Answer: Not if used correctly
- 329. • Is this extremely dangerous to use in your home?
- 330. • Are these extremely dangerous to use in your home together? • Answer: Yes!
- 331. • NaOCl + 2NH3 --> 2NaONH3 + Cl2.
- 332. • NaOCl + 2NH3 --> 2NaONH3 + Cl2.
- 333. Covalently bonded. Copyright © 2010 Ryan P. Murphy
- 334. Covalently bonded. Copyright © 2010 Ryan P. Murphy
- 335. Covalently bonded. Copyright © 2010 Ryan P. Murphy
- 336. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff.
- 337. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20
- 338. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55
- 339. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 =
- 340. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35
- 341. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 342. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 343. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 344. Covalently bonded. Copyright © 2010 Ryan P. Murphy CH4 Methane Electron Negativity Diff. Hydrogen = 2.20 Carbon = 2.55 2.55 – 2.20 = .35 Differences 1.7 or greater, the bond is usually ionic, Differences Less than 1.7, the bond is usually covalent, Unless the difference is less than 0.5 the bond has some degree of polarity Differences of less than 0.5 are considered to be nonpolar.
- 345. They have a low density. Copyright © 2010 Ryan P. Murphy
- 346. They have a low density. Copyright © 2010 Ryan P. Murphy
- 347. They have a low density. Copyright © 2010 Ryan P. Murphy
- 348. They have a low density. Copyright © 2010 Ryan P. Murphy
- 349. • SPONCH elements are non-metals. Copyright © 2010 Ryan P. Murphy
- 350. • 25 of the 92 naturally occurring elements are essential for life. – - Copyright © 2010 Ryan P. Murphy
- 351. • 25 of the 92 naturally occurring elements are essential for life. – SPONCH elements are the most biologically important. Copyright © 2010 Ryan P. Murphy
- 352. • Organic Chemistry: The chemistry of carbon compounds.
- 353. • Organic Chemistry: The chemistry of carbon compounds. – Carbon is the duct tape of life. It holds everything together.
- 354. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 355. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 356. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 357. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 358. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 359. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 360. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 361. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 362. Percentage of SPONCH elements in living things. S. Sulfur Trace P. Phosphorus 1.0% O. Oxygen 65.0% N. Nitrogen 3.3% C. Carbon 18.5% H. Hydrogen 9.56% Other (Trace) 3.0% Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 363. • Activity! Please complete an animal graph of the data. – Percentages shown after instructions. Copyright © 2010 Ryan P. Murphy
- 364. Copyright © 2010 Ryan P. Murphy
- 365. Copyright © 2010 Ryan P. Murphy
- 366. Copyright © 2010 Ryan P. Murphy
- 367. Copyright © 2010 Ryan P. Murphy
- 368. Copyright © 2010 Ryan P. Murphy
- 369. Copyright © 2010 Ryan P. Murphy
- 370. Copyright © 2010 Ryan P. Murphy
- 371. Copyright © 2010 Ryan P. Murphy
- 372. • Percentage of SPONCH elements in living things. • S. Sulfur Trace • P. Phosphorus 1.0% • O. Oxygen 65.0% • N. Nitrogen 3.3% • C. Carbon 18.5% • H. Hydrogen 9.56% • Other (Trace) 3.0% • Sulfur, Sodium, Magnesium, Copper, Zinc, Selenium, Molybdenum, Fluorine, Chlorine, Iodine, Manganese, Cobalt, Iron Lithium, Strontium, Aluminum, Silicon, Lead, Vanadium, Arsenic, Bromine Copyright © 2010 Ryan P. Murphy
- 373. • Activity! Malleable and Ductile vs. Brittle Copyright © 2010 Ryan P. Murphy
- 374. • Activity! Malleable and Ductile vs. Brittle – Please draw picture of each object. Copyright © 2010 Ryan P. Murphy
- 375. • Activity! Malleable and Ductile vs. Brittle – Please draw picture of each object. – Bend each one and label which was malleable and which was brittle. Copyright © 2010 Ryan P. Murphy
- 376. • Activity! Malleable and Ductile vs. Brittle – Please draw picture of each object. – Bend each one and label which was malleable and which was brittle. – Which one was a non-metal, Why? Copyright © 2010 Ryan P. Murphy
- 377. • Answer! The wood was a non-metal because it…? Copyright © 2010 Ryan P. Murphy
- 378. • Answer! The wood was a non-metal because it…? • - Isn’t malleable or ductile • - Doesn’t have luster / shine • - Doesn’t conduct heat or electricity Copyright © 2010 Ryan P. Murphy
- 379. • The paperclip is a metal because it… Copyright © 2010 Ryan P. Murphy
- 380. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 381. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 382. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 383. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 384. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 385. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 386. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 387. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 388. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 389. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 390. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 391. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 392. • The paperclip is a metal because it… – Is ductile and malleable, has luster, conducts heat, electricity, and has a high density, solid, and reactive. Copyright © 2010 Ryan P. Murphy
- 393. • Activity! Passing around the elements. – Wear goggles and gloves to be safe. – We will rotate the bags with the elements every minute. • (Do not remove from bag) – Feel free to hold bag to guess at density • (High, medium, low) – Record the name, and what family the element belongs to and then describe some of its physical properties. – Electrical conductivity will be collected at the end. – Word Bank: (high luster / dull) (Malleable / Brittle) (High density, Medium, Low density) (Conducts electricity / Does not conduct) (Solid / Gas) Copyright © 2010 Ryan P. Murphy
- 394. • Activity! Passing around the elements. – Wear goggles and gloves to be safe. – We will rotate the bags with the elements every minute. The names of the element will be on the bag. • (Do not remove from bag) – Feel free to hold bag to guess at density • (High, medium, low) – Record the name, and what family the element belongs to and then describe some of its physical properties. – Electrical conductivity will be gathered at the end. – Word Bank: (high luster / dull) (Malleable / Brittle) (High density, Medium, Low density) (Conducts electricity / Does not conduct) (Solid / Liquid / Gas) Copyright © 2010 Ryan P. Murphy
- 395. Copper: Conducts electricity, malleable, ductile, moderate luster, high density, solid Carbon (Charcoal), Doesn’t conduct electricity, low density, brittle, no luster, solid Sulfur: Doesn’t conduct electricity, low density, brittle, no luster, solid Silicon: High luster, medium density, brittle, solid. Aluminum: High luster, malleable, medium density, solid. Nickel: High luster, malleable, high density, solid. Lead: Some luster, malleable, very high density, solid Oxygen: Gas Iodine: In bottle (liquid) Example…
- 396. Copper: Conducts electricity, malleable, ductile, moderate luster, high density, solid Carbon (Charcoal), Doesn’t conduct electricity, low density, brittle, no luster, solid Sulfur: Doesn’t conduct electricity, low density, brittle, no luster, solid Silicon: High luster, medium density, brittle, solid. Aluminum: High luster, malleable, medium density, solid. Nickel: High luster, malleable, high density, solid. Lead: Some luster, malleable, very high density, solid Oxygen: Gas Iodine: In bottle (liquid) Example…
- 397. • Activity! Passing around the elements. – Wear goggles and gloves to be safe. – We will rotate the bags with the elements every minute. The names of the element will be on the bag. • (Do not remove from bag) – Feel free to hold bag to guess at density • (High, medium, low) – Record the name, and what family the element belongs to and then describe some of its physical properties. – Electrical conductivity will be gathered at the end. – Word Bank: (high luster / dull) (Malleable / Brittle) (High density, Medium, Low density) (Conducts electricity / Does not conduct) (Solid / Liquid / Gas) Copyright © 2010 Ryan P. Murphy
- 398. • Possible Elements – Copper: Conducts electricity, malleable, ductile, moderate luster, high density, solid – Carbon (Charcoal), Doesn’t conduct electricity, low density, brittle, no luster, solid – Sulfur: Doesn’t conduct electricity, low density, brittle, no luster, solid – Silicon: High luster, medium density, brittle, solid. – Aluminum: High luster, malleable, medium density, solid. – Nickel: High luster, malleable, high density, solid. – Lead: Some luster, malleable, very high density, solid – Oxygen: Gas – Iodine: In bottle (liquid) – Zinc: Luster, malleable, medium density – Magnesium: Luster, malleable, medium density Copyright © 2010 Ryan P. Murphy
- 399. • Activity! Elements and their density. Copyright © 2010 Ryan P. Murphy
- 400. • Activity! Elements and their density. – Goggles and Gloves please. – Avoid breathing in any dust or particles. – Step 1 • Zero scale with graduated cylinder. • Fill small graduated cylinder with a metal 10 ml. Repeat process for each. • Metals are…Copper BB’s, Aluminum Foil, Lead / Steel Split Shot (Fishing). • Weigh graduated cylinder to determine weight of each. Copyright © 2010 Ryan P. Murphy
- 401. • Activity! Elements and their density. • Fill graduated cylinder with water to the very top. • Gently pour in metal and collect the water displaced. • Add displaced water into another graduated cylinder and record in cm3 the volume of water. Repeat for each sample. • Find the density of each. • Density = Mass divided by volume. • Answer is in g/cm3 Copyright © 2010 Ryan P. Murphy
- 402. • Activity! Metals and Non-metals / Semimetals Copyright © 2010 Ryan P. Murphy
- 403. • Lab Suggestion! Metals, Non-metals, and Metalloids. • http://www.nclark.net/MetalNonmetalLab.htm • http://mrbentleycod.tripod.com/sitebuildercon tent/sitebuilderfiles/student- metalnonmetalmetalloidlab.pdf
- 404. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) – Which elements had properties of more than one group? – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium.
- 405. • Laboratory Investigation: Metals, Non- Metals, and Metalloids.
- 406. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid)
- 407. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 408. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 409. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 410. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 411. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 412. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 413. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Zinc Carbon Aluminum Copper
- 414. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Zinc Carbon Aluminum Copper
- 415. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 416. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Without looking at a periodic table, put the elements that we investigated into the correct category. (Metal, Non-metal, Metalloid) Metal Non-Metal Metalloid Magnesium Sulfur Silicon Zinc Carbon Aluminum Copper
- 417. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Which elements had properties of more than one group?
- 418. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Which elements had properties of more than one group? – Answer: Silicon had properties of metals and non-metals. It was like a metal because it conducted electricity and had luster. It was like a non-metal because it was brittle.
- 419. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium.
- 420. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium.
- 421. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. We know what happened to these?
- 422. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. We know what happened to these?
- 423. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. We know what happened to these?
- 424. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. We know what happened to these?
- 425. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. We know what happened to these?
- 426. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. – Answers:
- 427. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. – Answers: • Calcium: Metal, Reactive with Acid, luster, conductive. Similar to Magnesium
- 428. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. – Answers: • Calcium: Metal, Reactive with Acid, luster, conductive. Similar to Magnesium • Cadmium: Metal, reactive with acid, conductive, similar to Zinc.
- 429. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. – Answers: • Calcium: Metal, Reactive with Acid, luster, conductive. Similar to Magnesium • Cadmium: Metal, reactive with acid, conductive, similar to Zinc. • Selenium: Non-metal, brittle, poor conductor. Similar to Sulfur.
- 430. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. – Answers: • Calcium: Metal, Reactive with Acid, luster, conductive. Similar to Magnesium • Cadmium: Metal, reactive with acid, conductive, similar to Zinc. • Selenium: Non-metal, brittle, poor conductor. Similar to Sulfur.
- 431. • Laboratory Investigation: Metals, Non- Metals, and Metalloids. – Predict the physical and chemical properties of Calcium, Cadmium, and Selenium. – Answers: • Calcium: Metal, Reactive with Acid, luster, conductive. Similar to Magnesium • Cadmium: Metal, reactive with acid, conductive, similar to Zinc. • Selenium: Non-metal, brittle, poor conductor. Similar to Sulfur. Elements in the same family group behave similar. They have the same number of valence electrons.
- 432. • . Similar Family groups behave the same?
- 433. • You should be close to this question in your bundle. Copyright © 2010 Ryan P. Murphy
- 435. • The Noble Gases Copyright © 2010 Ryan P. Murphy
- 436. • The Noble Gases Copyright © 2010 Ryan P. Murphy
- 437. • Who are Nobles, Describe them? Copyright © 2010 Ryan P. Murphy
- 438. • Who are Nobles, Describe them? Copyright © 2010 Ryan P. Murphy
- 439. • Who are Nobles, Describe them? Copyright © 2010 Ryan P. Murphy Nobles: Possessing hereditary rank in a political system or social class derived from a feudalistic stage during a country's development.
- 440. Activity! A dramatic story about Noble Gases and Alkali Metals. – Story of woe and deception. – Includes a love theme. – Struggle between the classes. – Conspiracy. – Murder Plot. – Seduction. – Valence Electrons. Copyright © 2010 Ryan P. Murphy
- 441. I need Characters. Henry and Martha Copyright © 2010 Ryan P. Murphy
- 442. • I need Characters: Harold and Marcus Copyright © 2010 Ryan P. Murphy
- 443. • Will these two groups hang out socially? – Lets find out! Copyright © 2010 Ryan P. Murphy
- 444. “Hey Henry.” Copyright © 2010 Ryan P. Murphy
- 445. “Look at those incomplete orbitals over there.” Copyright © 2010 Ryan P. Murphy
- 446. “Maybe we should invite them to our family group.” Copyright © 2010 Ryan P. Murphy
- 447. “Oh no Marcus!” Copyright © 2010 Ryan P. Murphy
- 448. “I see those snooty noble gases again.” Copyright © 2010 Ryan P. Murphy
- 449. “Marcus!” Copyright © 2010 Ryan P. Murphy
- 450. “What is it Harold?” Copyright © 2010 Ryan P. Murphy
- 451. “I’m going to kill those two noble gases.” Copyright © 2010 Ryan P. Murphy
- 452. “Oh Martha!” Copyright © 2010 Ryan P. Murphy
- 453. “They don’t even have a full outer shell.” Copyright © 2010 Ryan P. Murphy
- 454. “They are so poor.” Copyright © 2010 Ryan P. Murphy
- 455. “I don’t think they even own more than one valence electron.” Copyright © 2010 Ryan P. Murphy
- 456. “How many electrons do we have again…?” Copyright © 2010 Ryan P. Murphy
- 457. “Oh Henry.” Copyright © 2010 Ryan P. Murphy
- 458. “We have a full outer shell.” Copyright © 2010 Ryan P. Murphy
- 459. “I don’t want any more electrons.” Copyright © 2010 Ryan P. Murphy
- 460. “So we don’t need them for anything.” Copyright © 2010 Ryan P. Murphy
- 461. “Harold!” Copyright © 2010 Ryan P. Murphy
- 462. “Don’t be a wicked atom.” Copyright © 2010 Ryan P. Murphy
- 463. “I hope your not getting all unstable again.” Copyright © 2010 Ryan P. Murphy
- 464. “I’m going to kill those two noble gases.” Copyright © 2010 Ryan P. Murphy
- 465. “Ha-Ha-Ha.” Copyright © 2010 Ryan P. Murphy
- 466. “Those poor Alkali Metals.” Copyright © 2010 Ryan P. Murphy
- 467. “What a waste of particles.” Copyright © 2010 Ryan P. Murphy
- 468. “They have nothing to offer us, we have all the electrons we can handle.” Copyright © 2010 Ryan P. Murphy
- 469. “Oh Henry!” Copyright © 2010 Ryan P. Murphy
- 470. “Your so Noble.” Copyright © 2010 Ryan P. Murphy
- 471. “I love it when you talk all sub- atomic.” Copyright © 2010 Ryan P. Murphy
- 472. “Oh Martha!” Copyright © 2010 Ryan P. Murphy
- 473. “I love the way your electrons orbit.” Copyright © 2010 Ryan P. Murphy
- 474. “So full and stable.” Copyright © 2010 Ryan P. Murphy
- 475. “Marcus!” Copyright © 2010 Ryan P. Murphy
- 476. “I’m going to kill those two noble gases.” Copyright © 2010 Ryan P. Murphy
- 477. “Harold you fool.” Copyright © 2010 Ryan P. Murphy
- 478. “They can’t be reacted with.” Copyright © 2010 Ryan P. Murphy
- 479. “They have a full outer shell.” Copyright © 2010 Ryan P. Murphy
- 480. “I’m sick of them thinking they’re so good because they have a full outer shell.” Copyright © 2010 Ryan P. Murphy
- 481. “I’m going to kill those noble fools.” Copyright © 2010 Ryan P. Murphy
- 482. “How will you do it?” Copyright © 2010 Ryan P. Murphy
- 483. “I’m not sure yet, but I swear on my one electron they will die.” Copyright © 2010 Ryan P. Murphy
- 484. “Come on, I see the halogens, they can make us more stable.” Copyright © 2010 Ryan P. Murphy
- 485. “Death is coming in a hail of electrons.” Copyright © 2010 Ryan P. Murphy
- 486. “It’s time to die you noble fool!” Copyright © 2010 Ryan P. Murphy
- 487. Copyright © 2010 Ryan P. Murphy
- 488. Copyright © 2010 Ryan P. Murphy
- 489. Copyright © 2010 Ryan P. Murphy
- 490. Copyright © 2010 Ryan P. Murphy
- 491. Copyright © 2010 Ryan P. Murphy
- 492. Copyright © 2010 Ryan P. Murphy
- 493. Copyright © 2010 Ryan P. Murphy
- 494. Copyright © 2010 Ryan P. Murphy
- 495. Copyright © 2010 Ryan P. Murphy
- 496. Copyright © 2010 Ryan P. Murphy
- 497. Copyright © 2010 Ryan P. Murphy
- 498. Copyright © 2010 Ryan P. Murphy “Oh Harold my boy” “That didn’t even steal one electron from my outer shell.”
- 499. Copyright © 2010 Ryan P. Murphy “If I only had more electrons I would…”
- 500. Copyright © 2010 Ryan P. Murphy “Oh Henry.” “Are you okay.”
- 501. Copyright © 2010 Ryan P. Murphy “Oh Martha” “That wimpy Alkali- metal didn’t do a thing.”
- 502. Copyright © 2010 Ryan P. Murphy “Wait a minute.” “You two can’t bond with each other well.”
- 503. “Ohhhhhhhhh” “He’s right Henry.” “We will never really be united.” Copyright © 2010 Ryan P. Murphy
- 504. “Oh Martha” “I’m sorry that the truth about us has been exposed.” Copyright © 2010 Ryan P. Murphy
- 505. “Our love will never become a bond.” “Ohhhhhhhhhhhh” Copyright © 2010 Ryan P. Murphy
- 506. “Our love will never become a bond.” “Ohhhhhhhhhhhh” Copyright © 2010 Ryan P. Murphy
- 507. “Ohhhhhhhhhhhh” Copyright © 2010 Ryan P. Murphy
- 508. “Ohhhhhhhhhhhhhhhhhhhhhhhh” Copyright © 2010 Ryan P. Murphy
- 509. “Ohhhhhhhhhhhhhhhhhhhhhhhhhhhh hhhhhhhhhhhhhhhhhhhhhhhhhh” Copyright © 2010 Ryan P. Murphy
- 510. “Ohhhhhhhhhhhhhhhhhhhhhhhhhhhh hhhhhhhhhhhhhhhhhhhhhhhhhhhhh hhhhhhhhhhhhhhhhhhhhhhhhhhhhh hhhhhhhhhhhhhhhhhhhhhhhhh” Copyright © 2010 Ryan P. Murphy
- 511. A round of applause for our actors and actresses. Copyright © 2010 Ryan P. Murphy
- 512. Alkali Metals (One valence Electron) Copyright © 2010 Ryan P. Murphy Noble Gases (Full Outer Shell)
- 513. Alkali Metals (One valence Electron) Copyright © 2010 Ryan P. Murphy Noble Gases (Full Outer Shell)
- 514. Alkali Metals (One valence Electron) Copyright © 2010 Ryan P. Murphy Noble Gases (Full Outer Shell)
- 515. Alkali Metals (One valence Electron) Copyright © 2010 Ryan P. Murphy Noble Gases (Full Outer Shell)
- 516. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Noble Gases Copyright © 2010 Ryan P. Murphy
- 517. • All of the inert gases have full outer shells with eight electrons – What does this mean? Think octet rule. Copyright © 2010 Ryan P. Murphy
- 518. • The noble gases have a full outer shell of electrons. Copyright © 2010 Ryan P. Murphy
- 519. • The noble gases have a full outer shell of electrons. – They don’t want to gain or lose any of them so they don’t react with other atoms very well. Copyright © 2010 Ryan P. Murphy
- 520. • They are quite happy not reacting with other elements, because they have a full outer shell with 8 electrons. Copyright © 2010 Ryan P. Murphy
- 521. • Who is in the family of noble gases? Copyright © 2010 Ryan P. Murphy
- 522. • Who is in the family?
- 523. • Who is in the family? • Helium (He),
- 524. • Who is in the family? • Helium (He), Neon (Ne),
- 525. • Who is in the family? • Helium (He), Neon (Ne), Argon (Ar),
- 526. • Who is in the family? • Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr),
- 527. • Who is in the family? • Helium (He), Neon (Ne), Argon (Ar), Krypton (Kr), Xenon (Xe), and Radon (Rn) Learn more at… http://www.chem4kids.com/files/elem_noblegas.html
- 528. • Radon is a dangerous gas that occurs when rock decays. It is a good idea to have basements checked. Copyright © 2010 Ryan P. Murphy
- 529. • Radon is a dangerous gas that occurs when rock decays. It is a good idea to have basements checked. – Radon causes cancer. Copyright © 2010 Ryan P. Murphy
- 530. • Halogens 17 (Salt-former) G R O U P
- 531. • Halogens 17 (Salt-former) G R O U P
- 532. • Halogens 17 (Salt-former) – The halogens exist, at room temperature, in all three states of matter.
- 533. • Halogens 17 (Salt-former) – The halogens exist, at room temperature, in all three states of matter. – All halogens have 7 electrons in their outer shells. Very reactive!
- 534. – It is grouped with the alkali metals because it has a similar outer shell electron configuration as they do. Copyright © 2010 Ryan P. Murphy
- 535. • Hydrogen is an odd ball. – It is grouped with the alkali metals because it has a similar outer shell electron configuration as they do. Copyright © 2010 Ryan P. Murphy
- 536. • Hydrogen is an odd ball. Copyright © 2010 Ryan P. Murphy
- 537. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. Copyright © 2010 Ryan P. Murphy
- 538. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Copyright © 2010 Ryan P. Murphy
- 539. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Copyright © 2010 Ryan P. Murphy
- 540. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 541. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 542. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 543. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 544. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 545. • Hydrogen is an odd ball. – It’s grouped with the alkali metals because it has a similar outer shell electron configuration as they do. It’s not metal? Also needs one electron. Copyright © 2010 Ryan P. Murphy
- 547. “This is a wicked hard homework question on the next slide.”
- 549. “Can anyone in the class explain this cartoon?”
- 550. “Thought question?”
- 552. • Video! (Optional) The Halogens and some exciting chemical reactions. – http://www.youtube.com/watch?v=u2ogMUDBaf4
- 553. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Halogens Copyright © 2010 Ryan P. Murphy
- 554. • Other Metals-these elements are ductile and malleable (aqua)
- 555. • Other Metals-these elements are ductile and malleable (aqua)
- 556. • Video Link! Gallium. Other Metal. Has a very low melting point. – Vanishing Spoon Illusion: http://www.youtube.com/watch?v=Nzoi9m3t4uM
- 557. H He Li Be B C N O F Ne Na Mg Al Si P S Cl Ar K Ca Sc Ti Ga Ge As Se Br Kr Key: Other Metals Copyright © 2010 Ryan P. Murphy
- 558. • The Rare Earth Elements are made up of two series of elements, the Lanthanide and Actinide Series Copyright © 2010 Ryan P. Murphy
- 559. • The Rare Earth Elements are made up of two series of elements, the Lanthanide and Actinide Series Copyright © 2010 Ryan P. Murphy
- 560. • The Rare Earth Elements are made up of two series of elements, the Lanthanide and Actinide Series Copyright © 2010 Ryan P. Murphy
- 561. • These groups are put on the bottom so the periodic table will fit on one page. Copyright © 2010 Ryan P. Murphy
- 562. • These groups are put on the bottom so the periodic table will fit on one page. Copyright © 2010 Ryan P. Murphy
- 563. • These groups are put on the bottom so the periodic table will fit on one page. – They fit to the right of Barium and Radium. Copyright © 2010 Ryan P. Murphy
- 564. • Video Link! Periodic Table. – https://www.youtube.com/watch?v=fLSfgNxoVGk
- 565. • Video Link! Periodic Table Crash Course – Preview for language and content. – http://www.youtube.com/watch?v=0RRVV4Dio mg&list=PL8dPuuaLjXtPHzzYuWy6fYEaX9mQ Q8oGr
- 566. • Periodic Table Song. A Nice Review – https://www.youtube.com/watch?v=1PSzSTilu_s
- 567. • Video! Meet the Elements from TMBG – http://www.youtube.com/watch?v=Uy0m7jnyv6U
- 568. • Atomic Cosplay Contest Coming Soon. – Element Cosplay (Create composite sketch or dress-up (school dress code enforced) with hand made costume) – Must choose one element from the Periodic Table of Elements – You must support your character with at least 8 factoids / characteristics / uses of that element. – Be prepared to present. Presentation is a part of your grade so get creative. – Learn more about Cosplay at… http://en.wikipedia.org/wiki/Cosplay
- 569. • Periodic Table worksheet links. – http://ellerbruch.nmu.edu/classes/cs255w03/cs2 55students/jeericks/p10/worksheet.pdf – http://www.docbrown.info/page03/3_34ptable/PT introQ.htm – Organization Periodic Table Coloring and Questions – https://teacher.ocps.net/theodore.klenk/chem/uni t4makeup/Periodic%20Table%20%20MU.pdf – http://www.getbookee.org/periodic-table- answers-chemistry/
- 570. • Periodic Table Project – Each student is responsible for 1 or more elements. – Teacher to organize / assign elements. – Each student is responsible for at least one / two elements in row. Provide atomic number, amu, symbol, etc. – Should include drawing of element, protons, neutrons, electrons (shells). – Uses of elements, interesting facts, how it reacts, compounds. – Where is it on the periodic table..non-metal, metal, noble gas, alkali metal…etc. – Arranged / Taped in the correct order / laminated – Artistic in nature / pleasing to the eye. – Example can be found on the next slide. Copyright © 2010 Ryan P. Murphy
- 571. Sulfur 16.amu Sulfur has 16 Electrons and 16 Protons. It is a perfect element and usually doesn’t occur as an Isotope. This is a picture of Sulfur. It is a non-metal. It does not have luster, it is brittle, and does not conduct electricity. Sulfur is a part of gun powder and used in explosives. The ancient Chinese were among the first to discover it’s power. Sulfur is often found near volcanoes and can be collected in mines and sometimes at the surface. Your Name!Copyright © 2010 Ryan P. Murphy
- 573. • Interactive Periodic Table of the Elements • http://www.ptable.com/
- 574. • Other Project. Creating a large Poster. – Each student will receive a large poster board. – Teacher will assign everyone an element from The Periodic Table of the Elements. • You may get more than one. • Once completed we will travel to the gym or outside (if not windy) to arrange our giant Periodic Table. – Use black Gorilla tape or Duct Tape to Record the Atomic Symbol in the Middle of the Poster board. Capital letter first, lowercase second. – Use black marker or more tape to record the Atomic Number, Name, and amu. – See example on the next page, and put your name on the back. Information can be much like the prior project with uses, abundance, history, electron configuration, and much more placed on the poster.
- 575. Lithium 6.94 amu
