This document discusses buffer solutions and their properties. It begins by defining a buffer as a solution that resists changes in pH when small amounts of acid or base are added. It then describes the common types of buffer solutions and illustrates buffer action using acetic acid/sodium acetate as an example. The mechanism and function of acidic and basic buffers is explained. It also covers the Henderson-Hasselbalch equation for calculating buffer pH, buffer capacity, and important buffer systems in pharmaceutical and biological contexts like the bicarbonate buffer system in blood.

![Buffer Capacity
Prepared By: Md. Imran Nur Manik; B.Pharm; M.Pharm Page 2
manikrupharmacy@gmail.com; Lecturer; Department of Pharmacy; Northern University Bangladesh.
Mechanism/function of a buffer
Mechanism of a buffer solution may be described by the following explanations:
A) Operation of acidic buffer
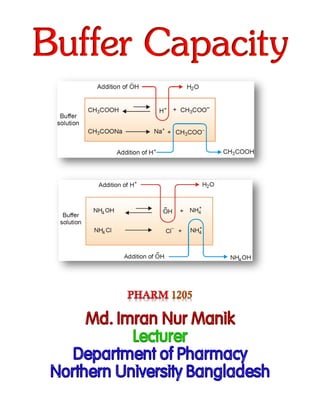
The function of an acidic buffer may be explained by considering a buffer solution composed of
CH3COONa and CH3COOH.
The pH of the buffer is governed by the equilibrium
CH3COOH⇌CH3COO–+H+ ……………………………………………………………… (1)
The buffer solution has a large excess of CH3COO– ions produced by complete ionization of sodium
acetate,
CH3COONa⇌CH3COO⎯+Na+……………………………………………………………. (2)
(1) Addition of HCl: Upon the addition of HCl, the increase of H+ ions is counteracted by association
with the excess of acetate ions to form unionized CH3COOH. Thus the added H+ ions are neutralized
and the pH of the buffer solution remains virtually unchanged. However owing to the increased
concentration of CH3COOH, the equilibrium (1) shifts slightly to the right to increase H+ ions. This
explains the marginal decrease of pH of the buffer solution on addition of HCl (Figure 2).
Fig.2. Mechanism of Buffer action of an acid buffer.
(2) Addition of NaOH: When NaOH is added to the buffer solution, the additional OH– ions combine
with H+ ions of the buffer to form water molecules. As a result the equilibrium (1) shifts to the right to
produce more and more H+ ions till practically all the excess OH– ions are neutralized and the original
buffer pH restored. However, a new equilibrium system is set up in which [CH3COOH] is lower than it
was in the original buffer. Consequently [H+] is also slightly less and pH slightly higher than the buffer
pH values (Figure 2).
Fact box: If the salt results from the reaction of a strong base and weak acid (e.g. sodium acetate from
reaction of sodium hydroxide and acetic acid), then the solution formed on hydrolysis will be basic, i.e.
CH3COOH+NaOH⇌ CH3COO⎯Na+
+ H2O
CH3COO⎯Na+
+ H2O ⇌ CH3COOH+OH⎯+Na+
Na+
does not react with water to any great extent, but CH3COO⎯is the conjugate base of the weak acid
CH3COOH and is therefore strong enough to react with water to produce OH⎯ions. The increase in
concentration of OH⎯gives a basic solution. These relationships can be summarised as follows:
Strong acid +Strong base Neutral salt
Strong acid+ Weak base Acidic salt
Weak acid + Strong base Basic salt
Weak acid + Weak base Neutral salt
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/buffermanikmergedfinalup-171021024552/85/Buffer-capacity-MANIK-3-320.jpg)
![Buffer Capacity
Prepared By: Md. Imran Nur Manik; B.Pharm; M.Pharm Page 3
manikrupharmacy@gmail.com; Lecturer; Department of Pharmacy; Northern University Bangladesh.
B) Operation of a Basic buffer:
The operation of a basic buffer may be explained by considering a buffer solution composed of NH4OH
and NH4Cl on the same lines as of an acid buffer (Figure 3). Upon the addition of HCl the H+ ions
combine with OH– ions of the buffer to form water molecules. The equilibrium, NH4OH⇌NH4
+ + OH–
is shifted to the right till all the additional H+ ions are neutralized and the original buffer pH restored.
When NaOH is added to the buffer solution, OH– ions associate with excess of NH4
+ ions to form
unassociated NH4OH. Thus the pH of the buffer is maintained approximately constant.
Fig.3. Mechanism of Buffer action of a basic buffer.
Calculation of the pH of Buffer Solutions
The pH of an acid buffer can be calculated from the dissociation constant, Ka, of the weak acid and the
concentrations of the acid and the salt used.
The dissociation expression of the weak acid, HA, may be represented as
HA⇌H++A–
...(1)..............................
][A
[HA]
][Hor
[HA]
]][A[H
and
Ka
Ka
The weak acid is only slightly dissociated and its dissociation is further depressed by the addition of the
salt (Na+A–) which provides A– ions (Common ion effect). As a result the equilibrium concentration of
the unionized acid is nearly equal to the initial concentration of the acid. The equilibrium concentration
[A–] is presumed to be equal to the initial concentration of the salt added since it is completely
dissociated. Thus we can write the equation (1) as-
...(2)..............................
[salt]
[acid]
][Hor
Ka
Where, [acid] is the initial concentration of the added acid and [salt] that of the salt used.
Taking negative logs on both sides of the equation (2), we have
)3.(........................................
[salt]
[acid]
loglog]log[H-
Ka
But, –log [H+]= pH and –log Ka = pKa
Thus from (3) we have
equation.HendersonsimplyorequationhHasselbalc-HendersonthecalledisiprelationshThis
[acid]
[salt]
logpHHence,
[acid]
[salt]
logpHor,
[salt]
[acid]
logpH
pKa
pKa
pKa
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/buffermanikmergedfinalup-171021024552/85/Buffer-capacity-MANIK-4-320.jpg)
![Buffer Capacity
Prepared By: Md. Imran Nur Manik; B.Pharm; M.Pharm Page 4
manikrupharmacy@gmail.com; Lecturer; Department of Pharmacy; Northern University Bangladesh.
In a similar way, the Henderson-Hasselbalch equation for a basic buffer can be derived. This can be
stated as:
[base]
[salt]
logHpHence, pKbO
Problem: Find the pH of a buffer solution containing 0.20 mole per litre CH3COONa and 0.15 mole
per litre CH3COOH. Ka for acetic acid is 1.8 10–5.
Problem: Calculate the concentration of acetic acid to be added to a 0.1 M solution of sodium acetate
to give a buffer of pH 5 (pKa of acetic acid=4.66).
Solution: Acetic acid is a weak acid, so its degree of ionisation is very small and the contribution to the
total concentration of acetate anions from ionisation of the acid can be ignored. The total salt
concentration is therefore 0.1 M from the fully ionised sodium acetate.
Using the Henderson–Hasselbalch equation
0.046M[acid]
[acid]
0.1
188.2
[acid]
0.1
log34.0
[acid]
0.1
log66.40.5
[acid]
[salt]
logpH
pKa
Problem: The Ka of propionic acid is 1.3410–5. What is the pH of a solution containing 0.5 M
propionic acid, C2H5COOH, and 0.5 sodium propionate, C2H5COONa. What happens to the pH of
this solution when volume is doubled by the addition of water?
Problem: A buffer solution contains 0.015 mole of ammonium hydroxide and 0.025 mole of
ammonium chloride. Calculate the pH value of the solution. Dissociation constant of NH4OH at the
room temperature is 1.8010–5.
Hints: Henderson-Hasselbalch equation for basic buffers is first used to determine the value of pOH.
[base]
[salt]
logHpHence, pKbO
Where Kb is the dissociation constant of the base.
We know that,
pH + pOH = 14.00
Thus, pH = 14.00 – pOH
Problem: Estimate the pH at 25°C containing 0.10 M sodium acetate and 0.03 M acetic acid pKa for
CH3COOH = 4.57.
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/buffermanikmergedfinalup-171021024552/85/Buffer-capacity-MANIK-5-320.jpg)
![Buffer Capacity
Prepared By: Md. Imran Nur Manik; B.Pharm; M.Pharm Page 5
manikrupharmacy@gmail.com; Lecturer; Department of Pharmacy; Northern University Bangladesh.
Buffer capacity
Buffer capacity is a measure of the efficiency of a buffer, in resisting changes in pH. The buffer capacity
is defined as the number of moles per litre of strong monobasic acid or base required to produce an
increase or decrease of one pH unit in the solution. It is also known as buffer efficiency, buffer index,
and buffer value.
Conventionally, the buffer capacity (β) is expressed as the amount of strong acid or base, in
gram-equivalents, that must be added to 1 liter of the solution to change its pH by one unit.
A buffer solution can resist a small amount of change of pH on adding acid or alkali to the solution.
Buffer capacities ranging from 0.01-0.1 are usually adequate for most pharmaceutical solutions. In
1922, Van Slyke first introduced an approximate equation to determine the buffer capacity by the
following equation:
)1.....(..............................
ΔpH
ΔB
β
In which β = Buffer capacity, delta Δ=a finite change, and ΔB =the small increment in gram equivalents
(gEq)/liter of strong base added to the buffer solution to produce a pH change of ΔpH. According to
equation (1), the buffer capacity of a solution has a value of 1 when the addition of 1 g. of strong base
(or acid) to 1 liter of the buffer solution results in a change of 1 pH unit.
The higher the buffer capacity the less the buffer solution changes its pH.
A more exact equation for buffer capacity:
The buffer capacity calculated from above equation is only approximate. It gives the average buffer
capacity over the increment of base added.
Koppel and Spiro and Van Slyke developed a more exact equation,
2
3
3
])[(
][
3.2
OHKa
OHKa
Cβ
Where, C = the total buffer concentration (i.e. the sum of the molar concentrations of acid and salt).
Buffers in pharmaceutical and biological systems
BUFFERS AND THE BODY:
Body fluids contain buffering agents and buffer systems that maintain pH at or near pH=7.4. Important
endogenous (natural) buffer systems include carbonic acid/sodium bicarbonate and sodium phosphate in
the plasma and haemoglobin, and potassium phosphate in the cells.
An in vivo value of pH < 6.9 or pH > 7.8 can be life threatening. Pharmaceutical solutions generally
have a low buffer capacity in order to prevent overwhelming the body’s own buffer systems and
significantly changing the pH of the body fluids. Buffer concentrations of between 0.05 and 0.5 M and
buffer capacities between 0.01 to 0.1 are usually sufficient for pharmaceutical solutions.
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/buffermanikmergedfinalup-171021024552/85/Buffer-capacity-MANIK-6-320.jpg)
![Buffer Capacity
Prepared By: Md. Imran Nur Manik; B.Pharm; M.Pharm Page 6
manikrupharmacy@gmail.com; Lecturer; Department of Pharmacy; Northern University Bangladesh.
USEFUL BUFFER MIXTURES
Components pH range
HCl, Sodium citrate 01 – 05
Citric acid, Sodium citrate 2.5 – 5.6
Acetic acid, Sodium acetate 3.7 – 5.6
K2HPO4, KH2PO4 5.8 – 08
Na2HPO4, NaH2PO4 06 – 7.5
Borax, Sodium hydroxide 9.2 – 11
KH2PO4 is the acid, with a pH in the range of 4.4–4.7. K2HPO4 has a pH of 8.0.
H2CO3⇌ H++HCO3
–
The pH of blood is controlled by a bicarbonate (H2CO3/HCO3
–) buffer system. When the pH gets too
high (high OH concentration), the OH– reacts with carbonic acid (H2CO3) to form HCO3
–) and H2O.
When the pH gets too low (high H+ concentration), the H+ reacts with HCO3
– to form H2CO3. Because
H2CO3 is a weak acid, the H+ stays associated with the H2CO3. Since pH is an important factor in
many physiological processes, a change in the blood pH is a potentially life threatening condition
requiring immediate regulation.
The HCO3
-/H2CO3 buffer system is present in blood in greatest concentration and is very important in
maintaining the pH of blood within normal limit. The concentration of HCO3
- and H2CO3 in blood are
0.02M and 0.00125M respectively and hence the [HCO3
-]/[H2CO3] ratio is 20/1. In blood, the pKa
value for first ionization stage at body temperature is 6.1.Thus the
calculation will be as
The phosphoric and protein buffers of plasma are of relatively little
important as compared with bicarbonate buffer in regulating pH.
7.4pHOr,
1.26.1pHOr,
1
20
log6.1pHOr,
[Acid]
[Salt]
logpKapH
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/buffermanikmergedfinalup-171021024552/85/Buffer-capacity-MANIK-7-320.jpg)
![Buffer Capacity
Prepared By: Md. Imran Nur Manik; B.Pharm; M.Pharm Page 7
manikrupharmacy@gmail.com; Lecturer; Department of Pharmacy; Northern University Bangladesh.
Importance of biological buffer
Buffer is very important for biological system. Some of the pictures are as follows
1. Buffer maintains constant [H+] in the body required for optimum cellular activity.
2. The pH of blood (around 7.4) is controlled by bicarbonate (H2CO3/HCO3
–) buffer system.
3. The phosphate buffer system (HPO4
2-/H2PO4
-) plays a role in plasma and erythrocytes.
H2PO4
-+H2O⇌H3O++HPO4
2-
M/A: Any acid reacts with monohydrogen phosphate to form dihydrogen phosphate
HPO4
2- + H3O+ H2PO4
- + H2O
monohydrogen phosphate dihydrogen phosphate
The base is neutralized by dihydrogen phosphate
H2PO4
- + OH- HPO4
2- + H3O+
dihydrogen phosphate monohydrogen phosphate
4. Proteins as a buffer: Proteins contain –COO- groups, which, like acetate ions (CH3COO-), can
act as proton acceptors. Proteins also contain –NH3
+ groups, which, like ammonium ions
(NH4
+), can donate protons.
M/A: If acid comes into blood, hydronium ions can be neutralized by the –COO- groups
-COO- + H3O+- COOH + H2O
If base is added, it can be neutralized by the –NH3
+ groups
-NH3
+ + OH-- NH2 + H2O
Md.
Imran
Nur
Manik](https://image.slidesharecdn.com/buffermanikmergedfinalup-171021024552/85/Buffer-capacity-MANIK-8-320.jpg)


