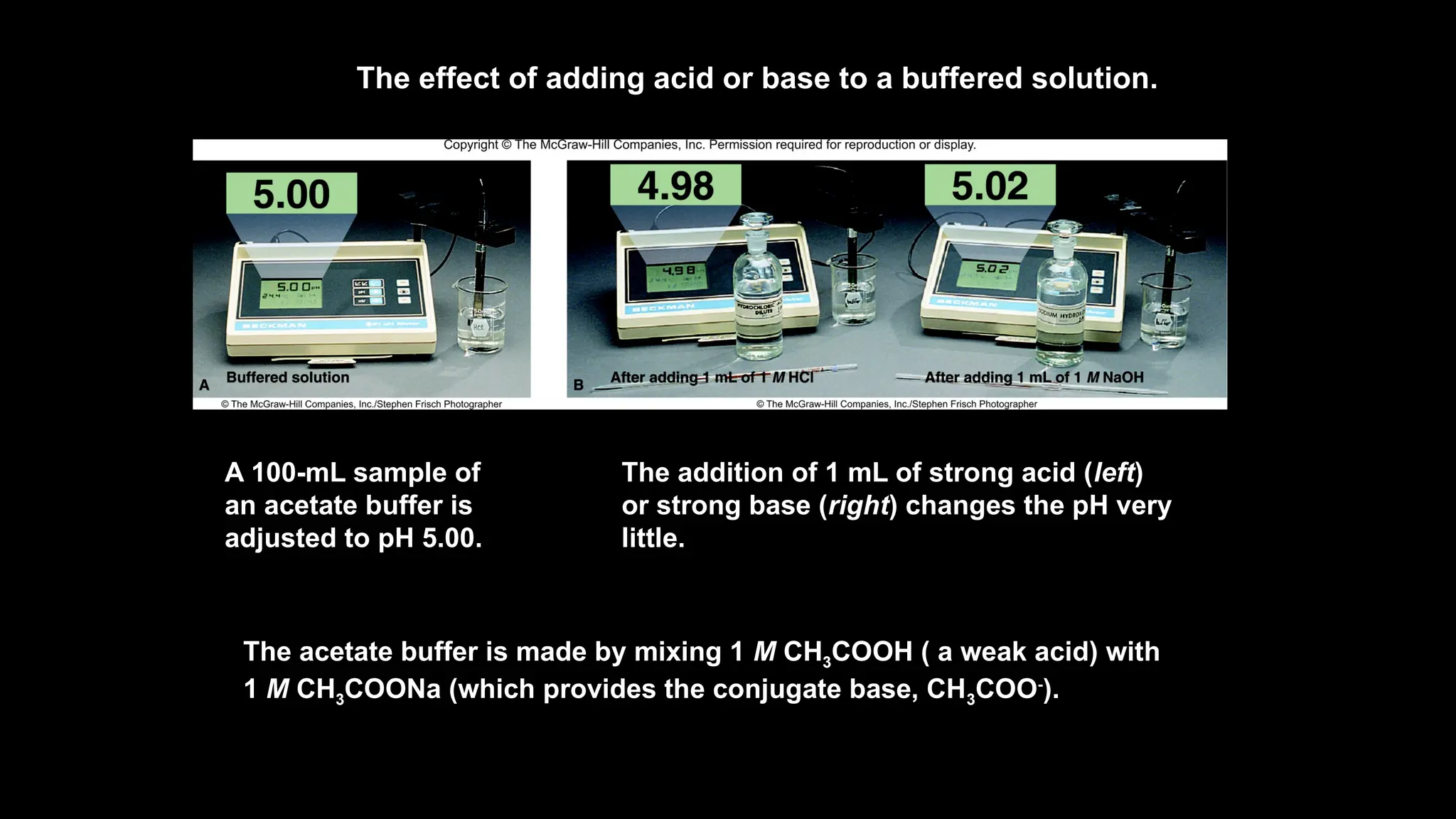
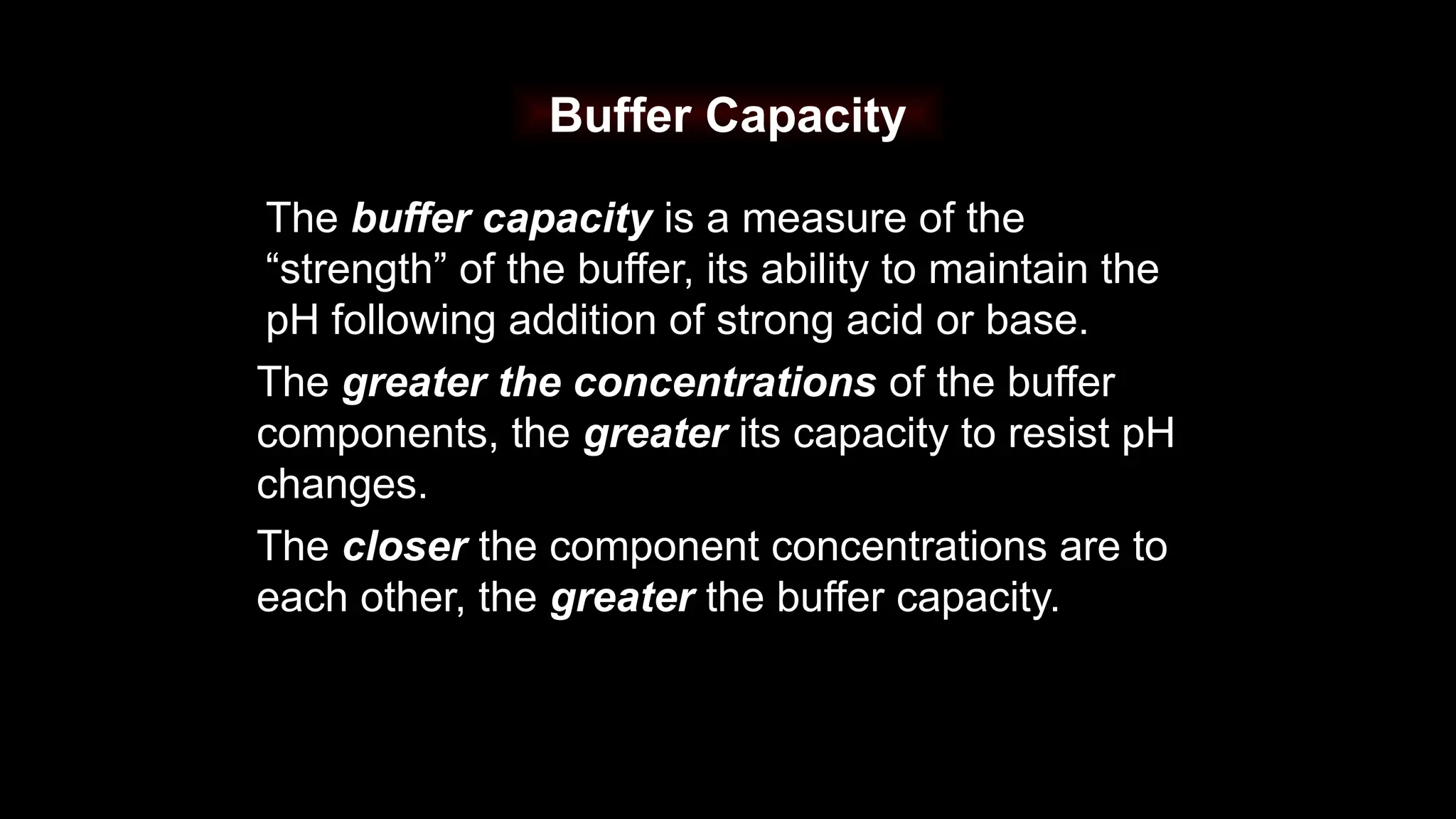
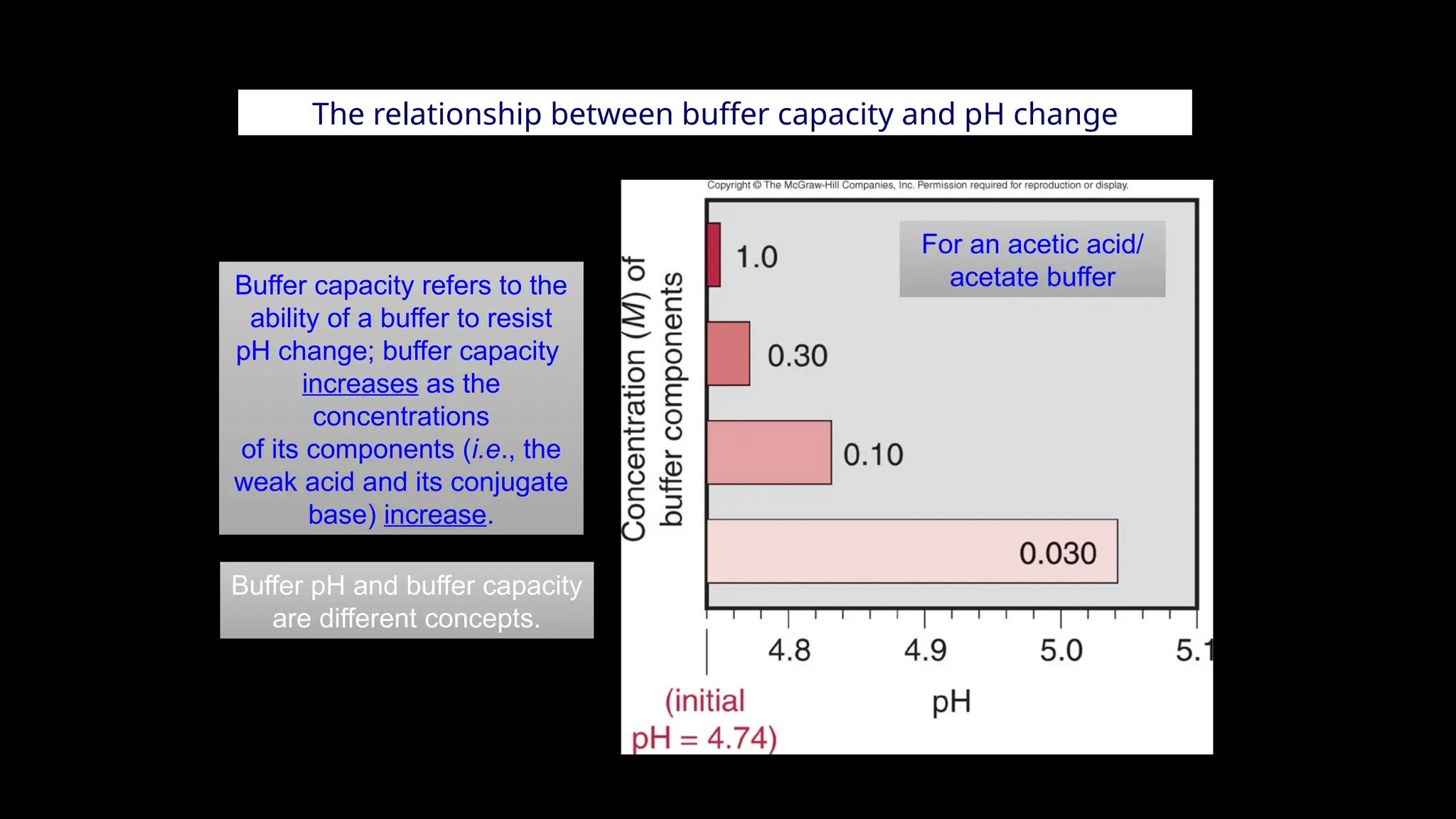
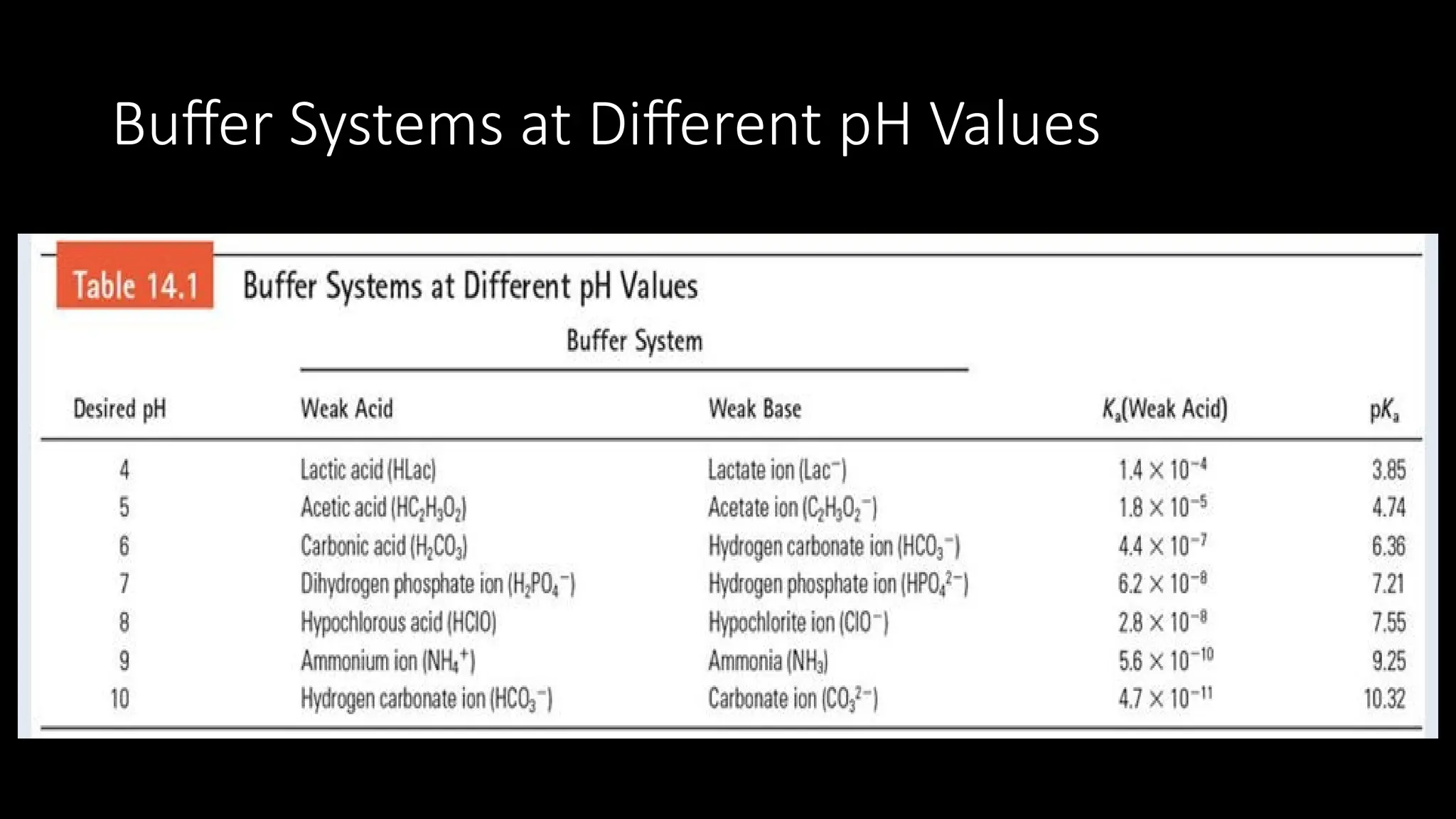
The document explains the concept of buffer solutions, which are composed of weak acid and its conjugate base and resist changes in pH when acids or bases are added. It describes how buffers work through equilibrium shifts and the common-ion effect, and outlines the Henderson-Hasselbalch equation for calculating pH of buffer solutions. Additionally, it discusses buffer capacity and its relationship with the concentrations of buffer components.





![Acid-Base Buffer
Systems
Buffers function by reducing changes in [H3O+
]
that result from additions of acid or base to the solution.
Buffers are composed of the conjugate
acid-base pair of a weak acid.
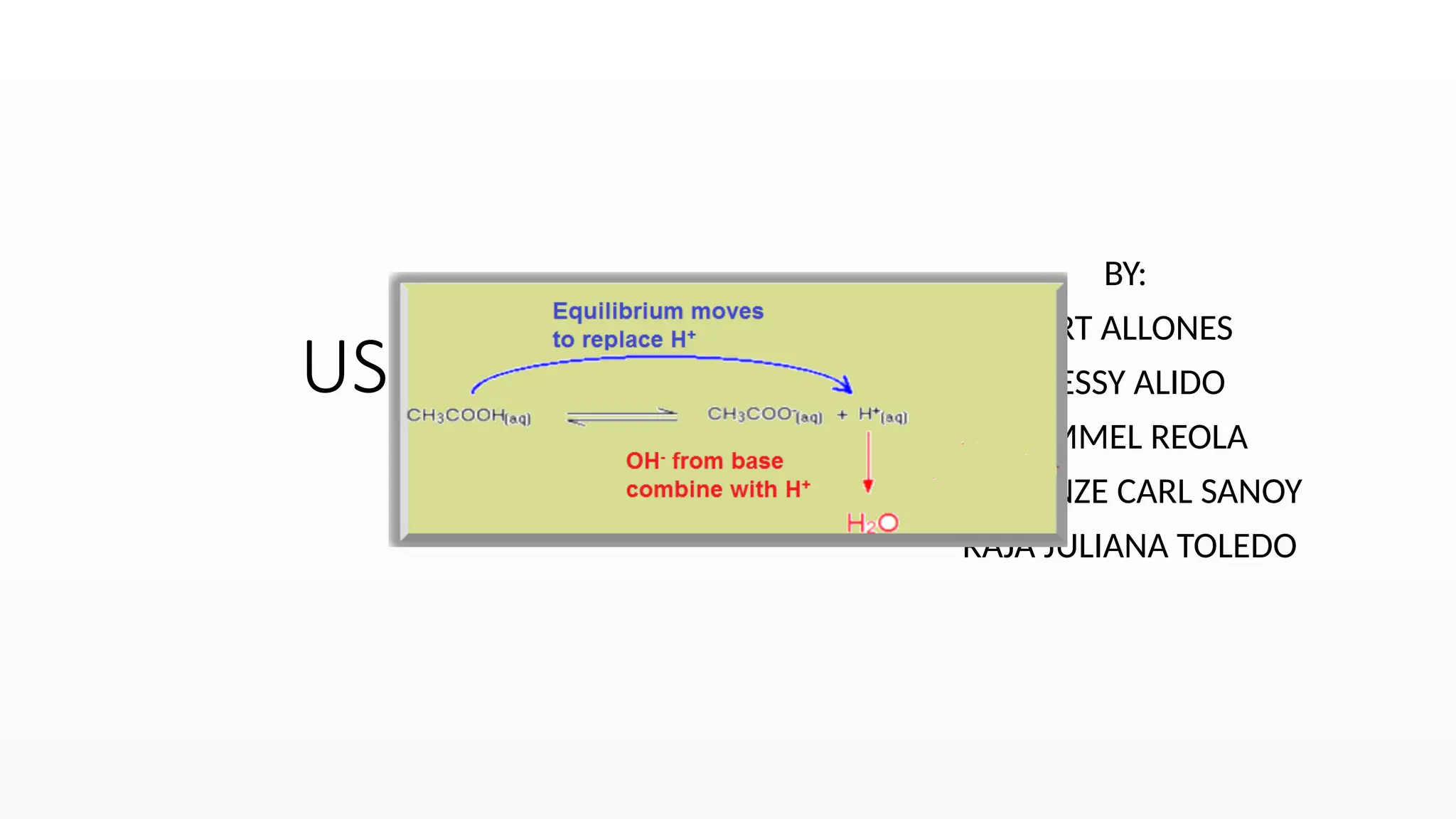
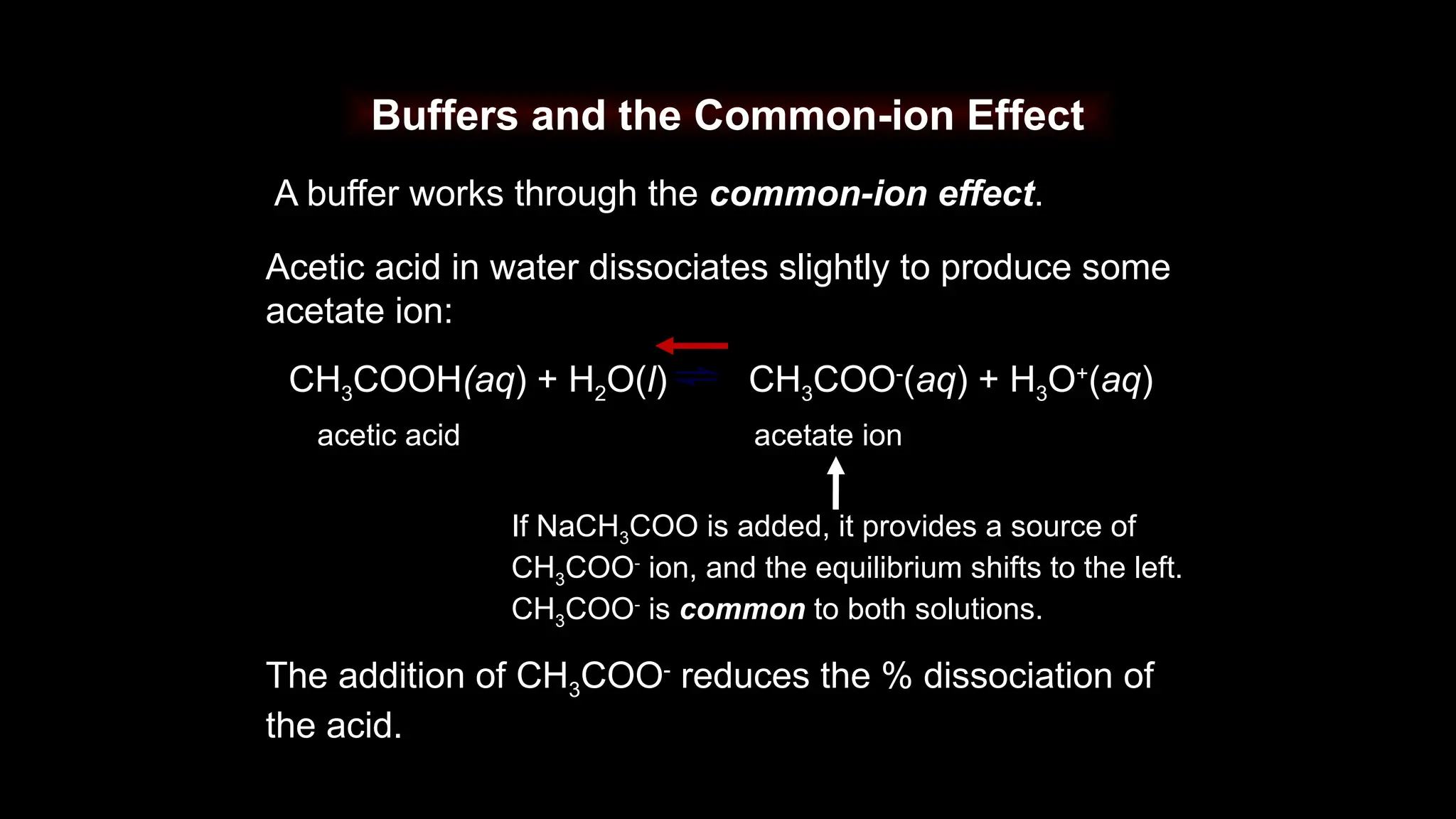
Buffers function via the common ion effect.
CH3COOH(aq) + H2O(l) CH3COO-
(aq) + H3O+
(aq)
The common ion effect occurs when a reactant containing
a given ion is added to an equilibrium mixture that already
contains that ion and the position of the equilibrium shifts
away from forming more of it.](https://image.slidesharecdn.com/useofbuffersolutions-241019150011-30902dce/75/buffer-solutions-report-presentation-pptx-9-2048.jpg)
![The Effect of Added Acetate Ion on the
Dissociation of Acetic Acid
[CH3COOH]init [CH3COO-
]added % Dissociation*
[H3O+
] pH
0.10 0.00 1.3 1.3x10-3
2.89
0.10 0.050 0.036 3.6x10-5
4.44
0.10 0.10 0.018 1.8x10-5
4.74
0.10 0.15 0.012 1.2x1015
4.92
*
% Dissociation =
[CH3COOH]dissoc
[CH3COOH]init
x 100](https://image.slidesharecdn.com/useofbuffersolutions-241019150011-30902dce/75/buffer-solutions-report-presentation-pptx-11-2048.jpg)
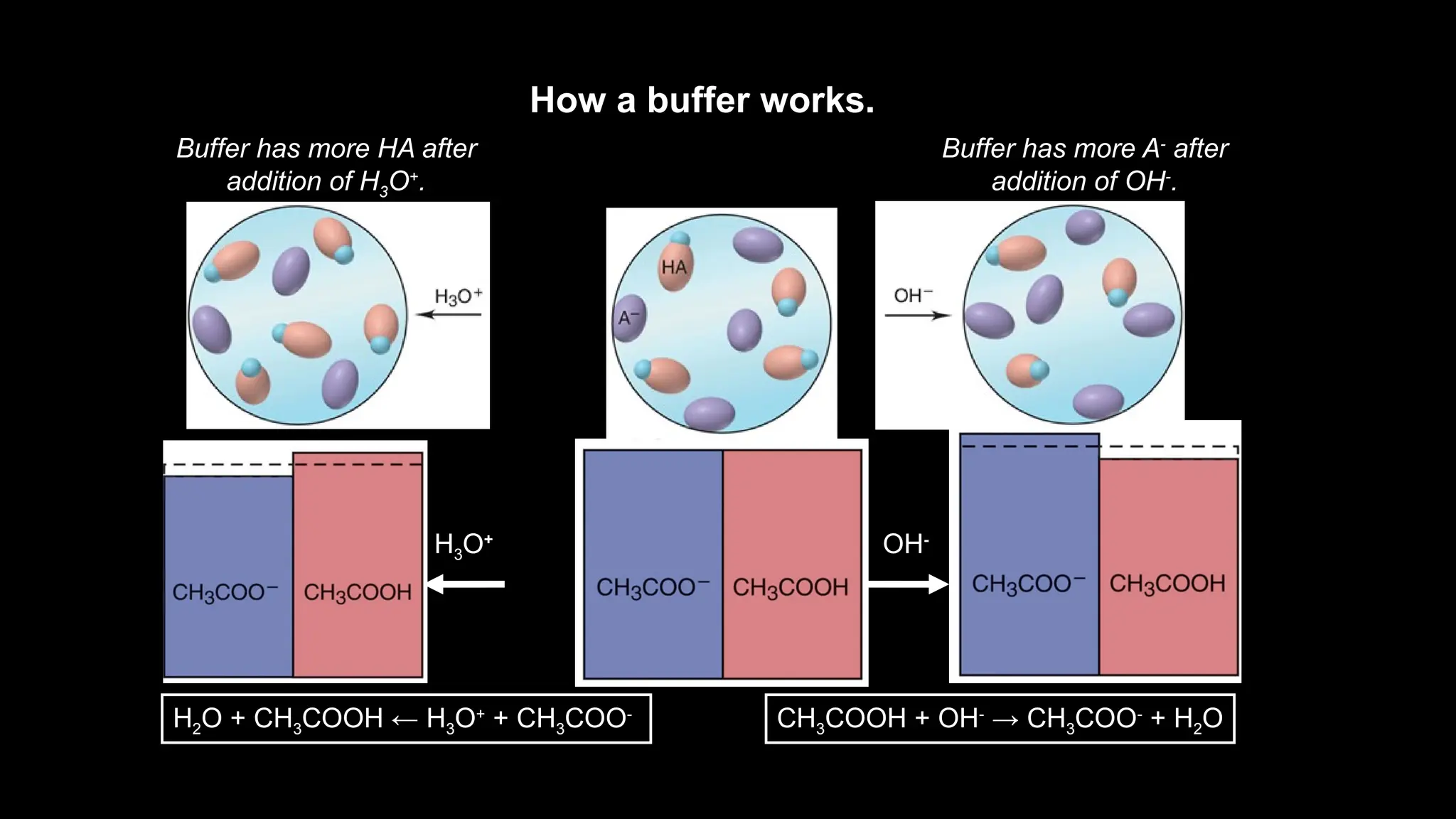
![How a Buffer Works
The buffer components (HA and A-
) are able to consume
small amounts of added OH-
or H3O+
by a shift in
equilibrium position.
CH3COOH(aq) + H2O(l) CH3COO-
(aq) + H3O+
(aq)
Added H3O+
reacts with
CH3COO-
, causing a
shift to the left.
Added OH-
reacts with
CH3COOH, causing a shift to
the right.
The shift in equilibrium position absorbs the change in
[H3O+
] or [OH-
], and the pH changes only slightly.](https://image.slidesharecdn.com/useofbuffersolutions-241019150011-30902dce/75/buffer-solutions-report-presentation-pptx-13-2048.jpg)
![The Henderson-Hasselbalch
Equation
An equation that relates pH, pKa and the ratio of
weak acid to its conjugate base for a buffer solution.
HA + H2O H3O+
+ A-
Ka = [H3O+
][A-
]/[HA]
[H3O+
] = Ka x [HA]/[A-
]
-log [H3O+
] = -log Ka - log ([HA]/[A-
])
pH = pKa + log ([A-
]/[HA])
pH = pKa + log ([base]/[acid])
Special case:
when [base] =
[acid], the pH of
the buffer
solution equals
the pKa of the
weak acid.
or](https://image.slidesharecdn.com/useofbuffersolutions-241019150011-30902dce/75/buffer-solutions-report-presentation-pptx-17-2048.jpg)

![SOLUTION:
Let a = [] at equilibrium
CH3COOH(aq) + H2O(l) CH3COO-
(aq) + H3O+
(aq)
Ka =
I 0.20 0 0
C - a +a +a
E 0.20 - a a a](https://image.slidesharecdn.com/useofbuffersolutions-241019150011-30902dce/75/buffer-solutions-report-presentation-pptx-19-2048.jpg)


















