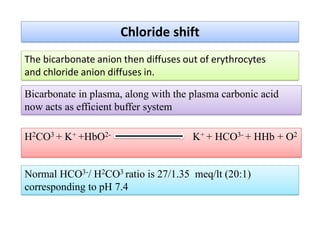
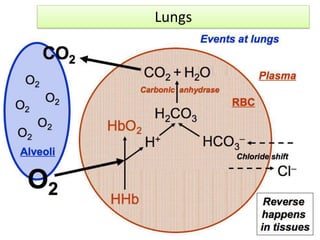
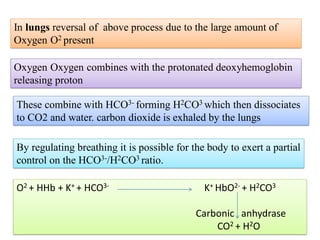
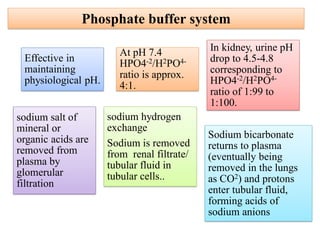
The document discusses physiological acid base balance and buffer systems that maintain pH levels in the human body. It covers three main points:
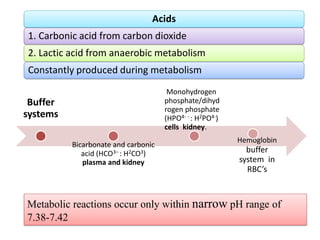
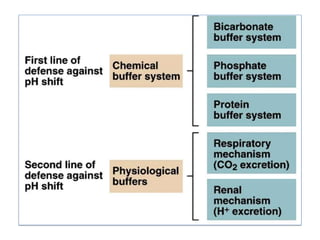
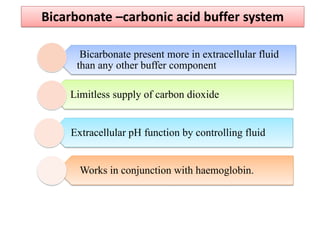
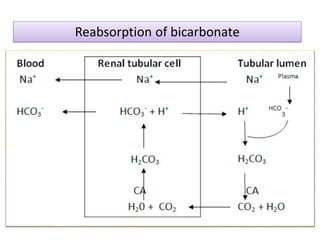
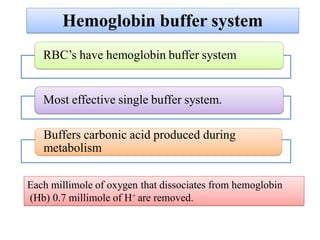
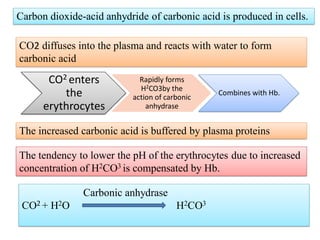
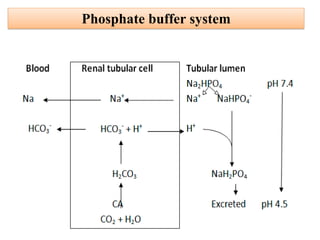
1. It describes the bicarbonate-carbonic acid buffer system that functions to regulate extracellular pH levels through the lungs, plasma, and kidneys. This system works together with the hemoglobin buffer system in red blood cells.
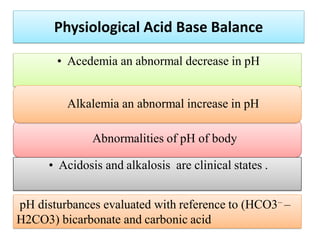
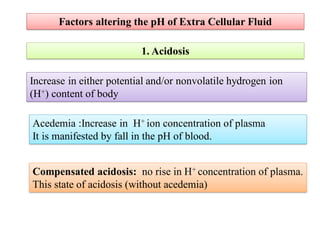
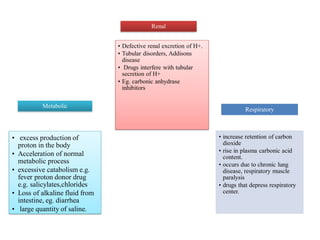
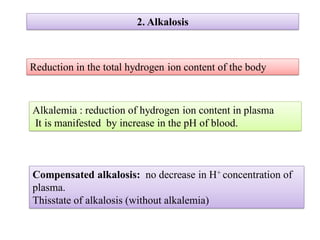
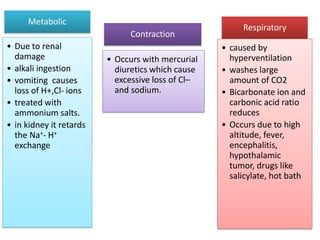
2. It explains factors that can cause acidosis or alkalosis, including metabolic, respiratory, and renal causes that affect hydrogen ion concentrations and pH levels. Compensated and uncompensated forms of acidosis and alkalosis are also discussed.
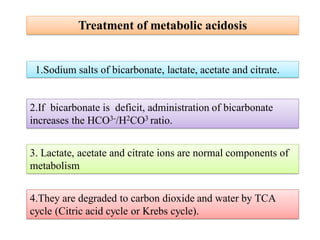
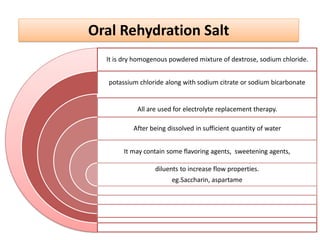
3. Treatment methods for metabolic acidosis include administration of sodium salts of bicar