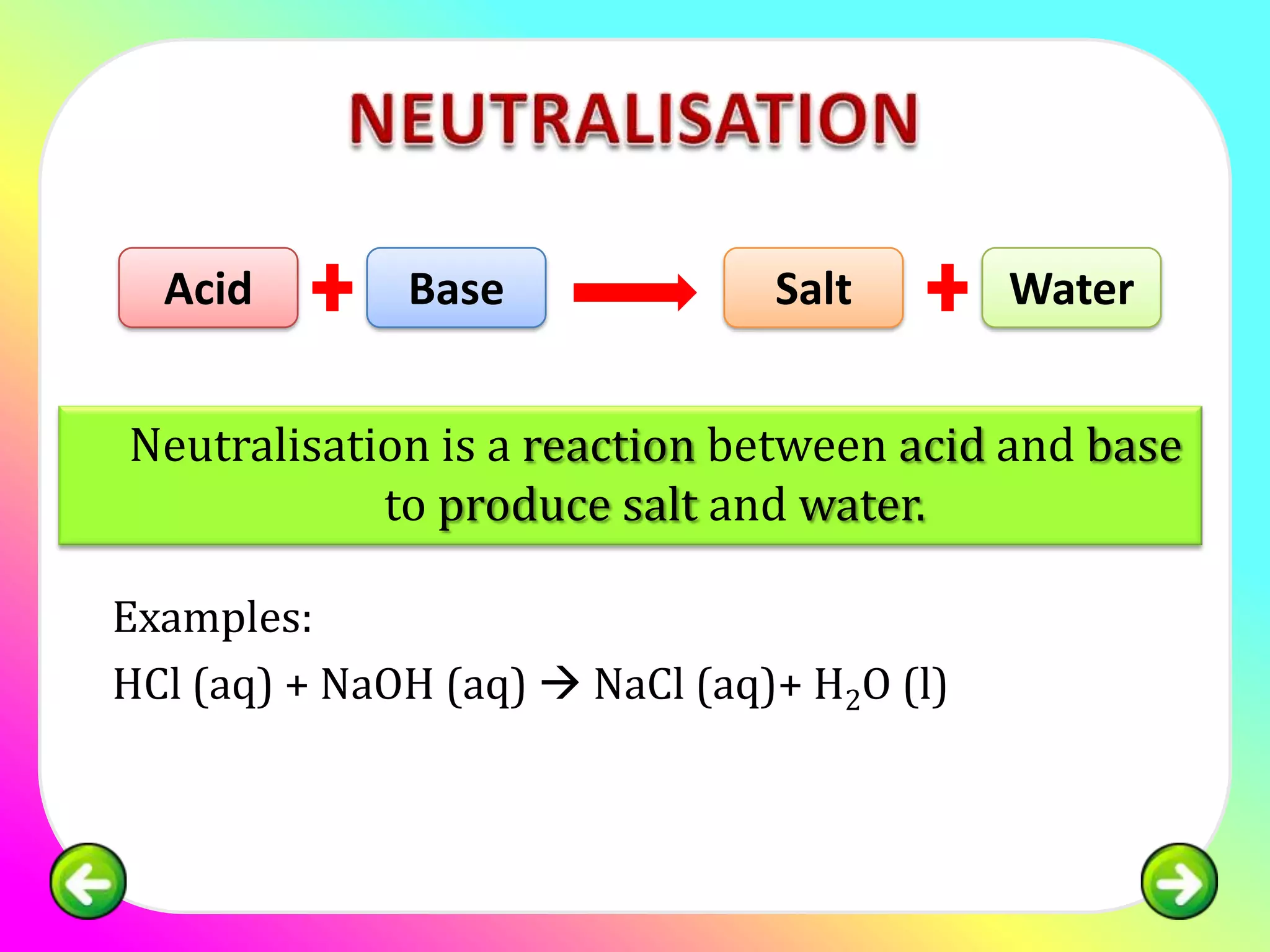
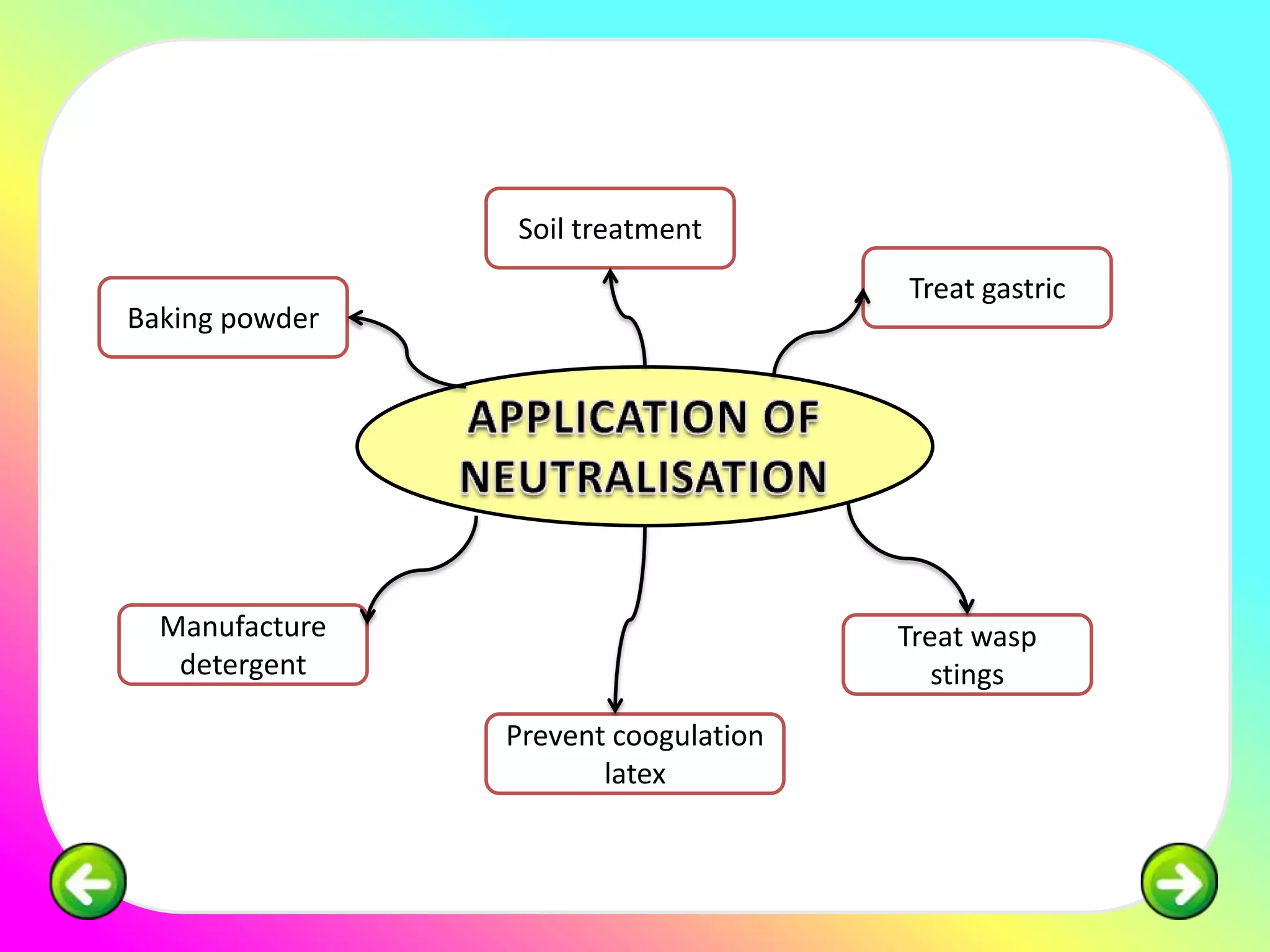
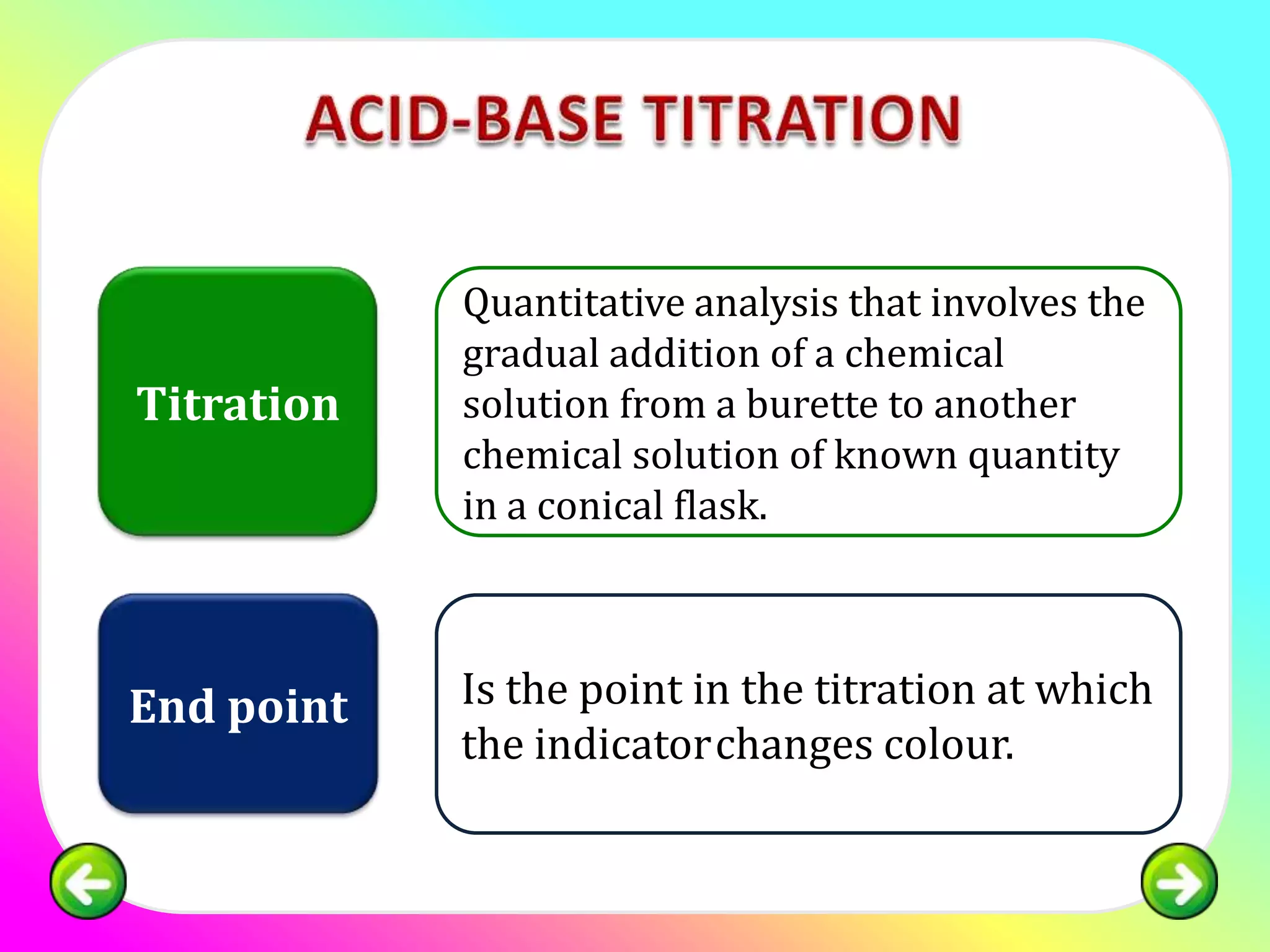
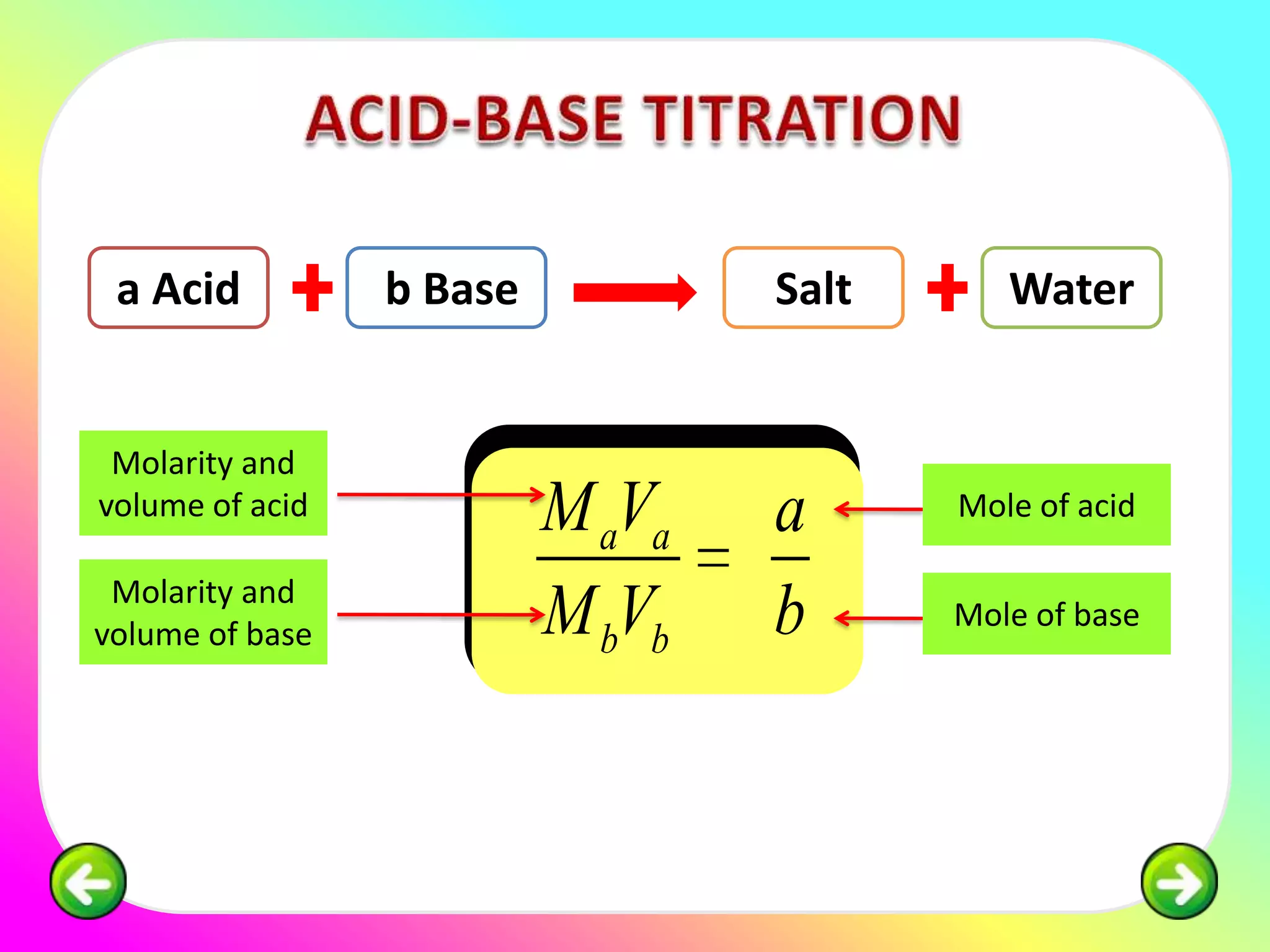
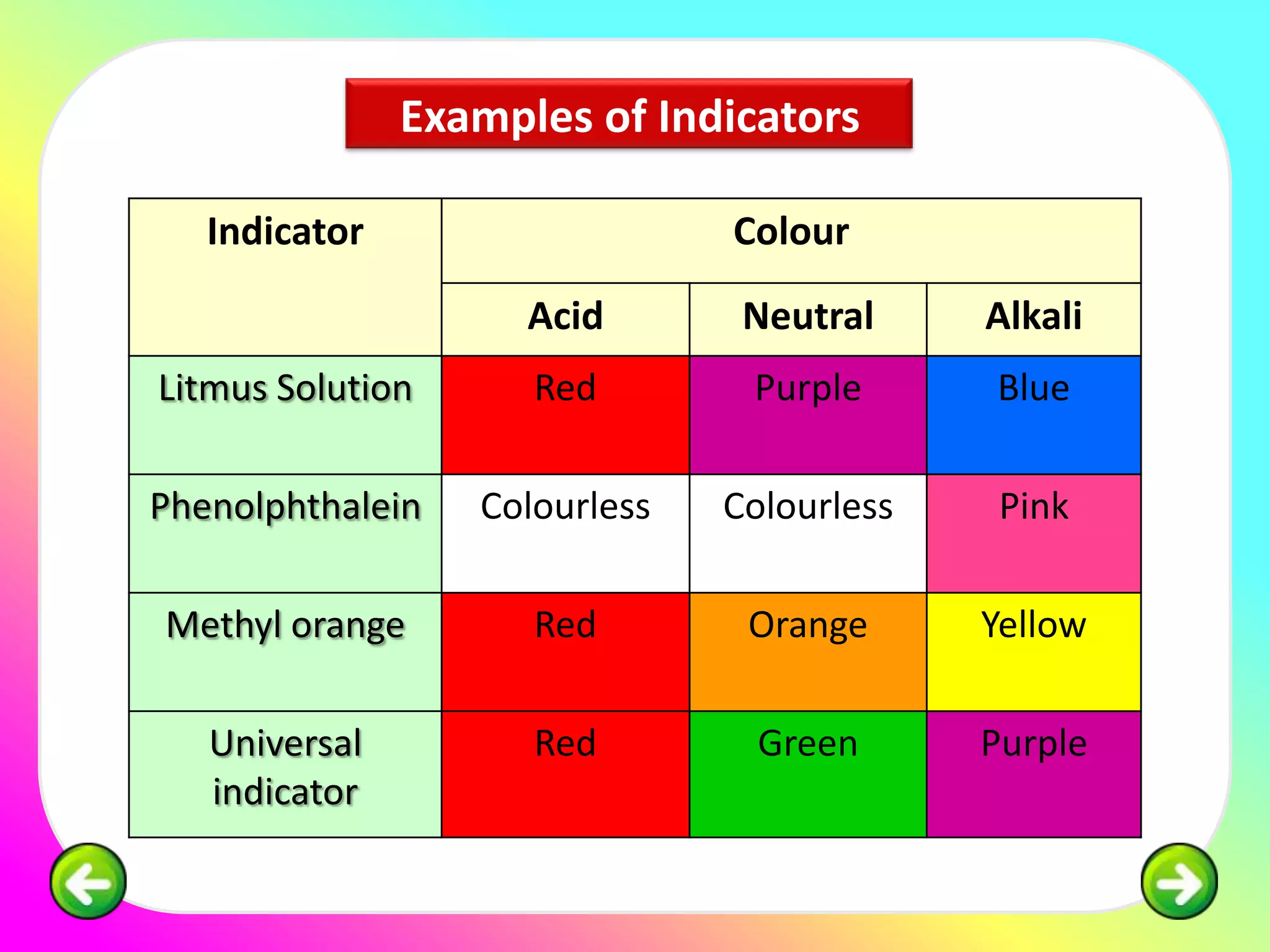
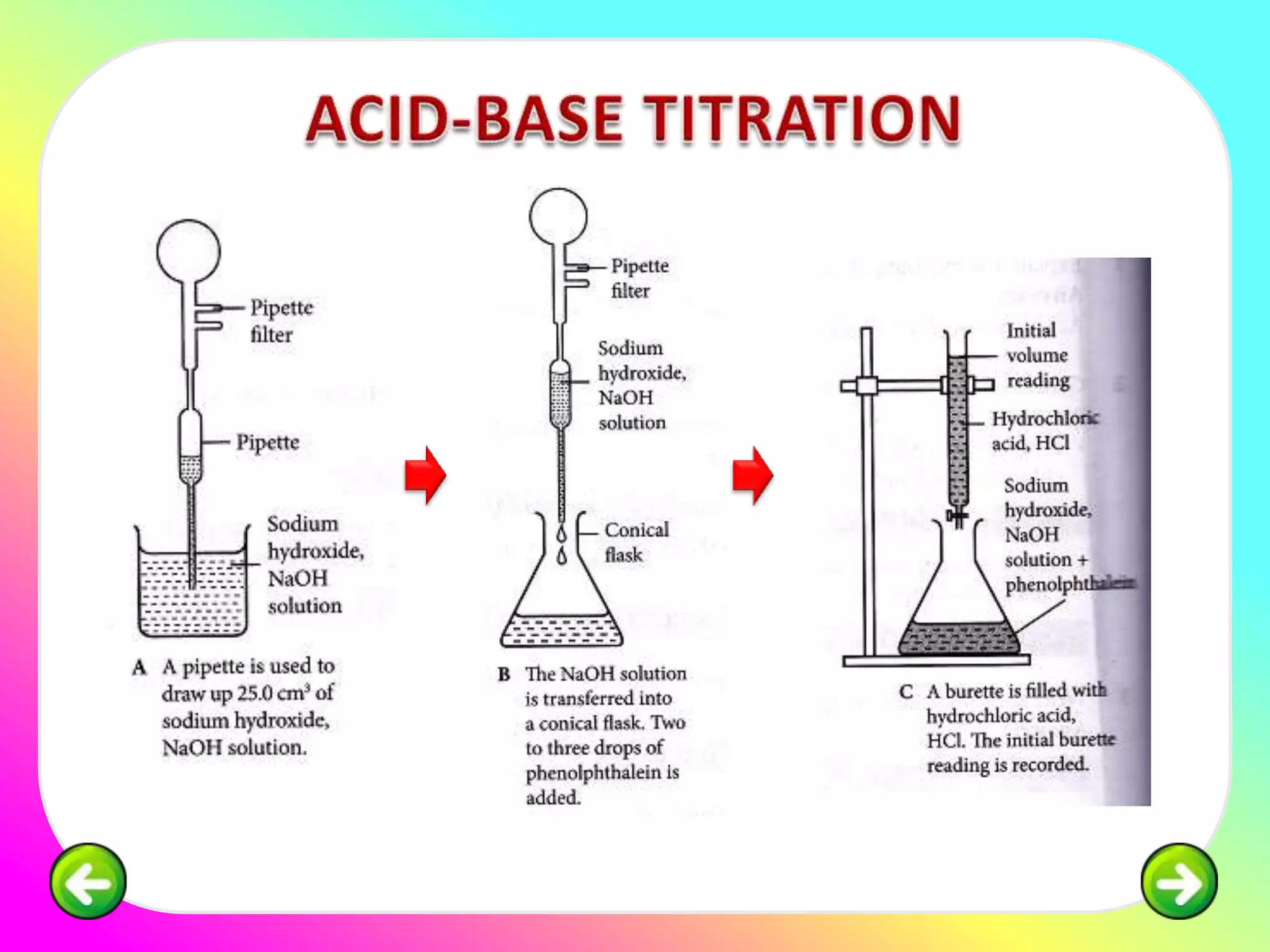
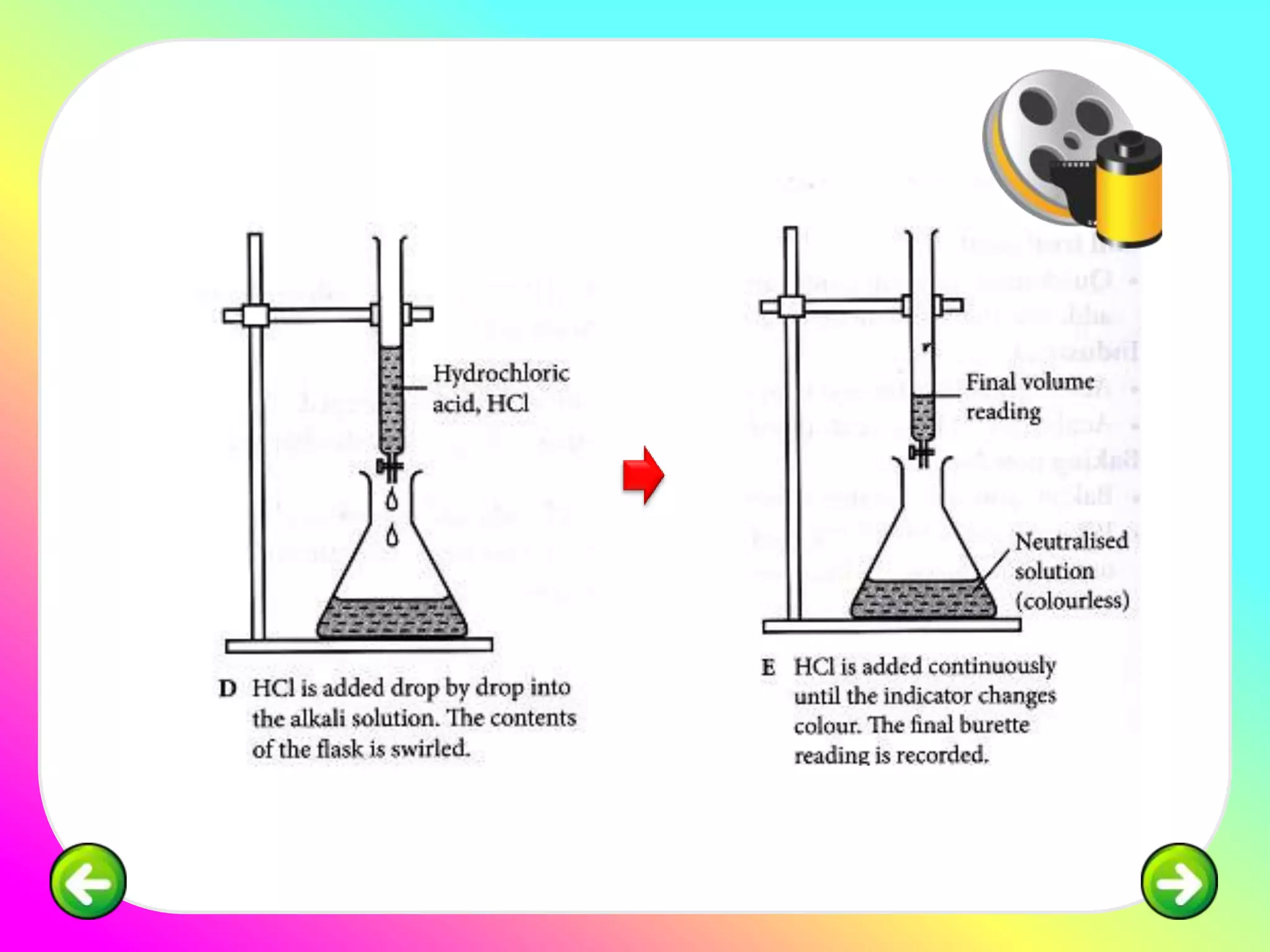
The document discusses neutralization reactions and acid-base titrations. It explains that neutralization is a reaction between acids and bases that produces salts and water. It provides examples of neutralization equations. It also describes acid-base titrations as a quantitative analysis involving gradually adding an acid or base from a burette to determine the endpoint, or point at which the indicator changes color. The document provides practice problems involving using molarity, volumes, and balanced equations to calculate concentrations or volumes in neutralization reactions.


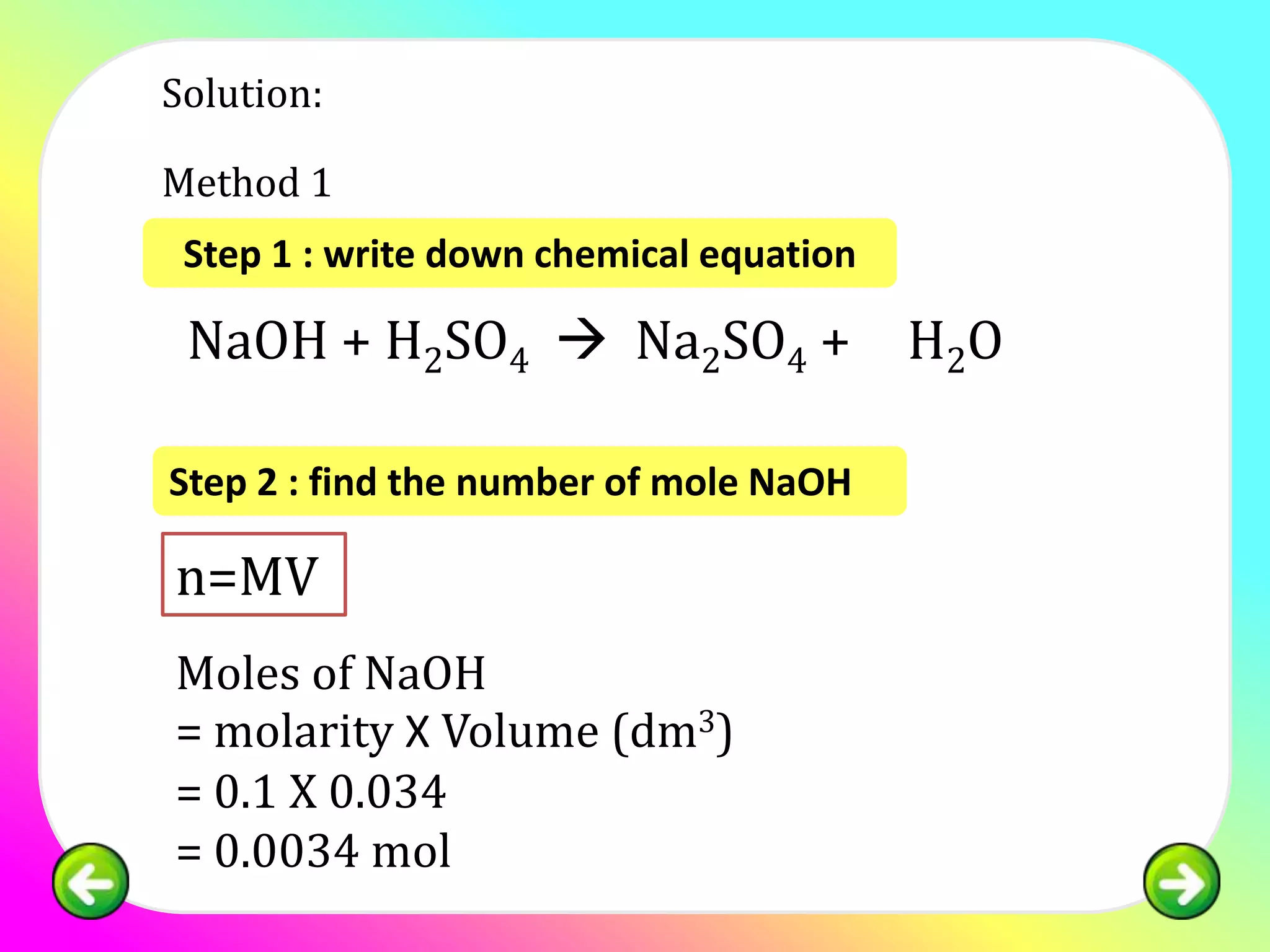
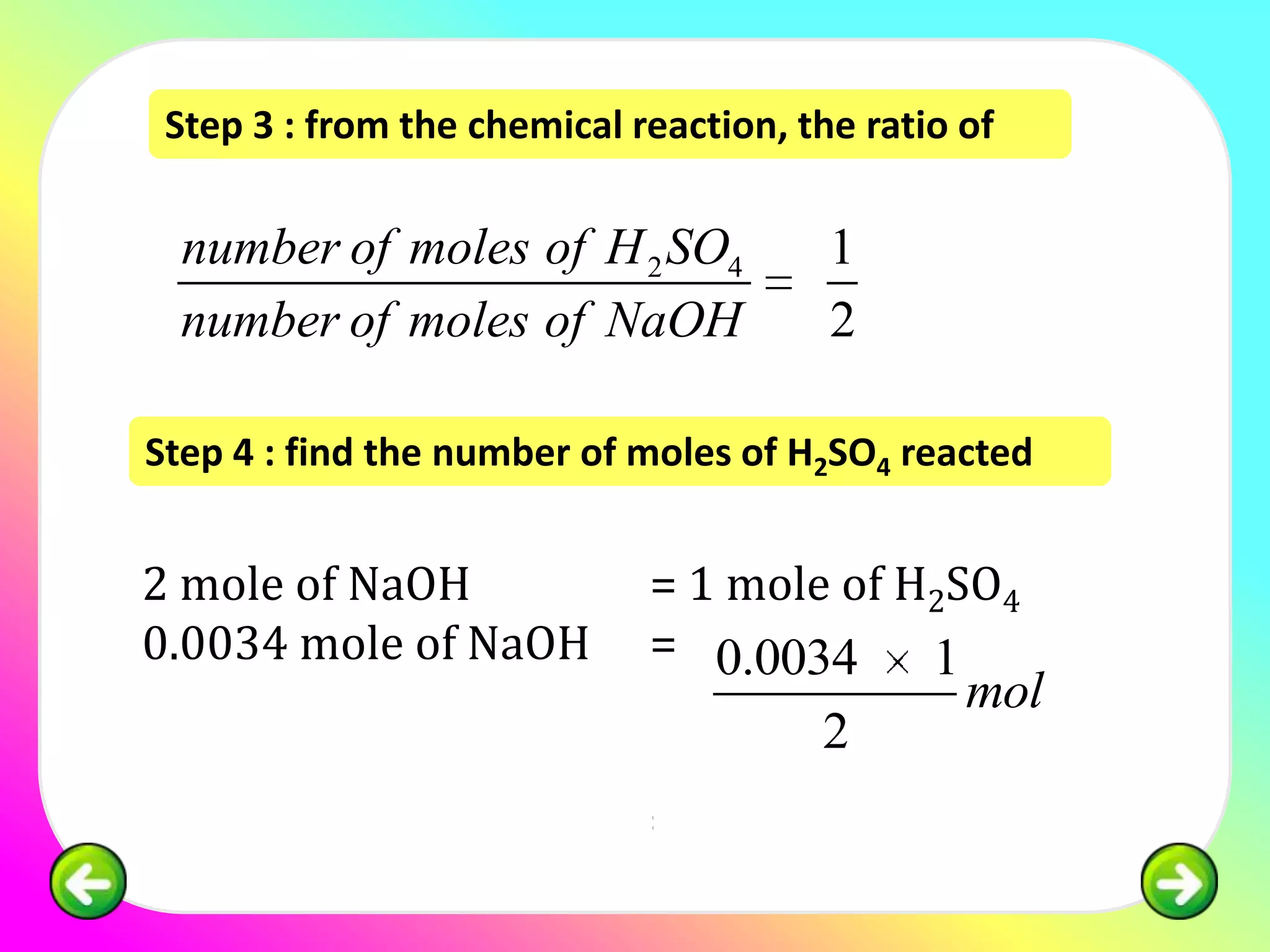
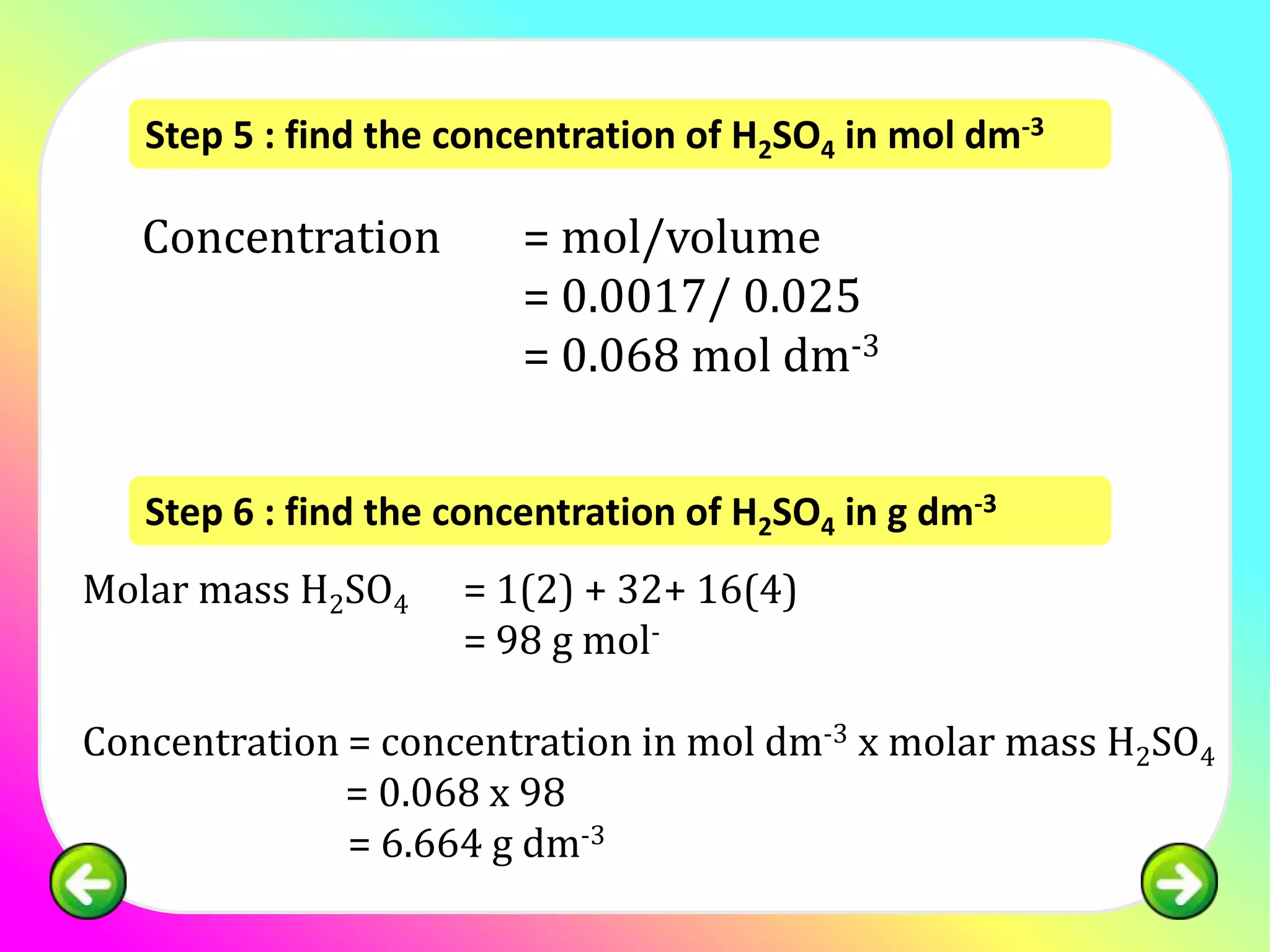
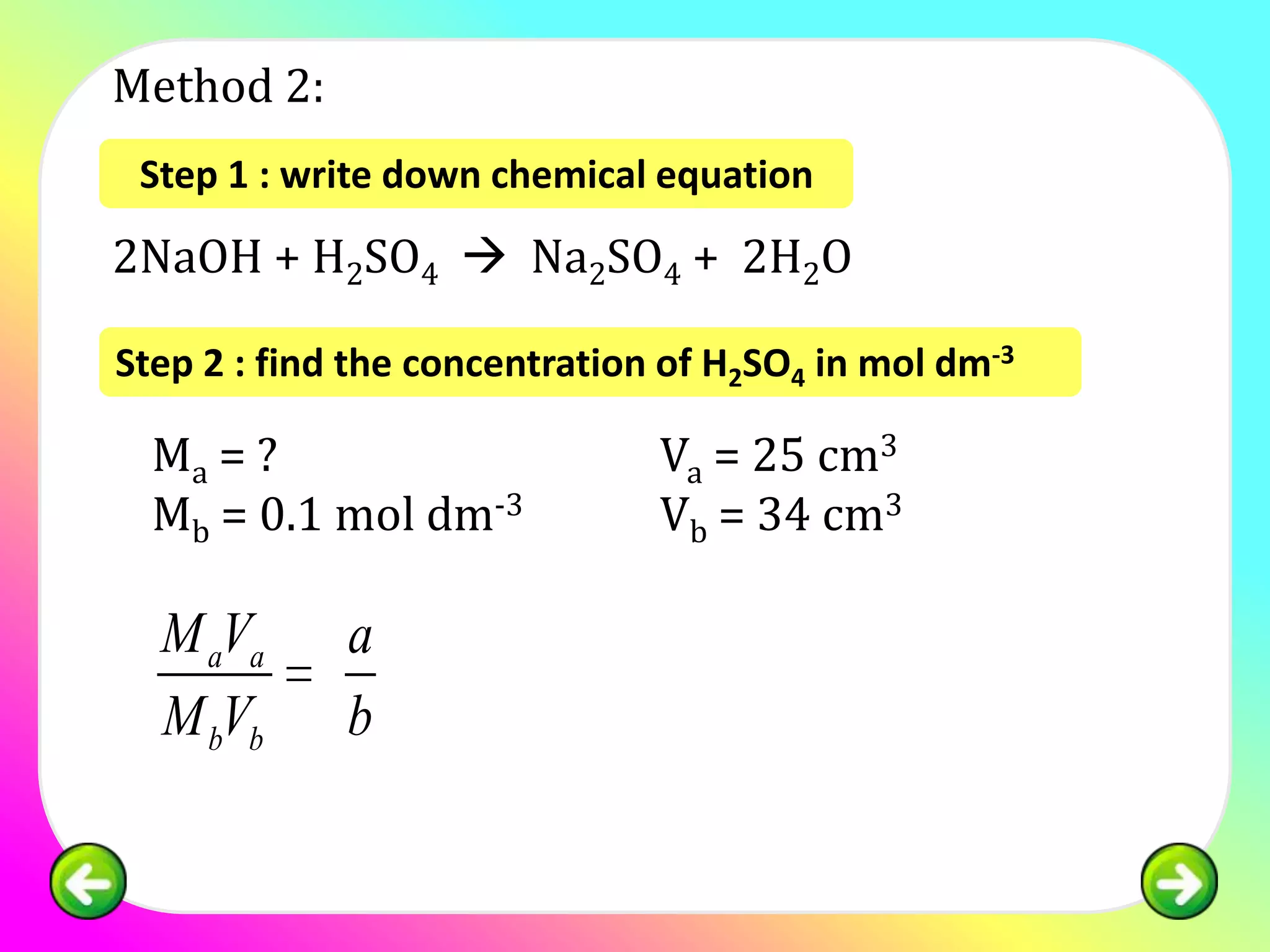
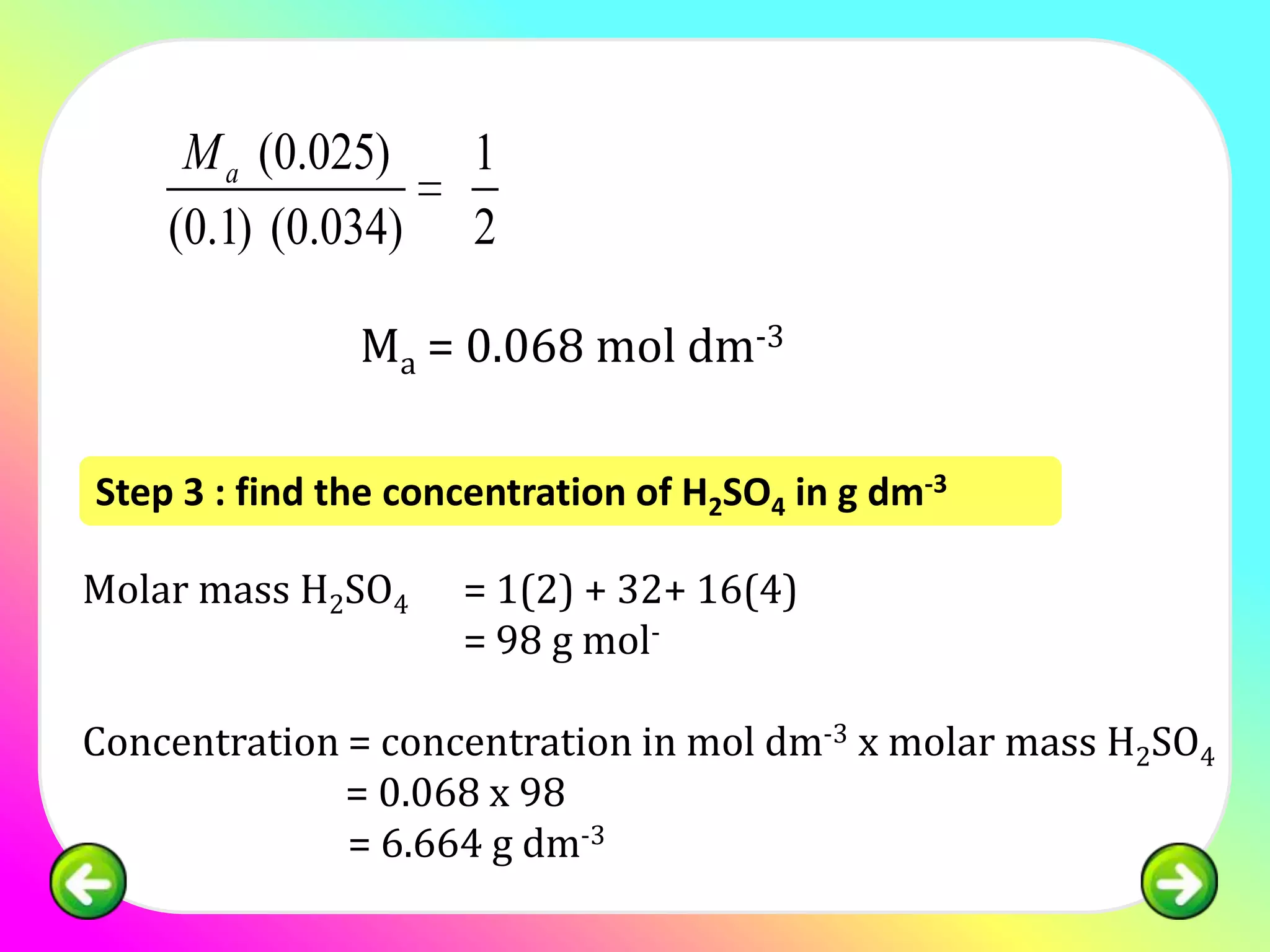
![Question 1:
25.0 cm3 of sulphuric acid is neutralised by 34.0
cm3 of 0.1 mol of dm-3 NaOH. Calculate the
concentration of sulphuric acid in:
(a) mol dm -3
(b) g dm-3
[relative atomic mass; H:1, S:32, O:16]](https://image.slidesharecdn.com/chapter7acidbasespart4-130226002935-phpapp02/75/Chapter-7-acid-bases-part-4-11-2048.jpg)
![Question 2:
What volume of 0.20 mol dm-3 nitric
acid is required to neutralise 0.14 g of
potassium hydroxide? [relative atomic
mass: O: 16, K:39, H:1]
Ans: 12.5 cm3/0.0125 dm3](https://image.slidesharecdn.com/chapter7acidbasespart4-130226002935-phpapp02/75/Chapter-7-acid-bases-part-4-17-2048.jpg)

