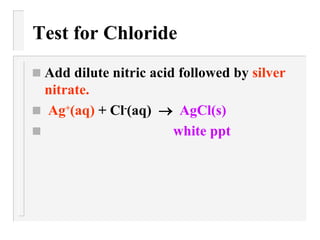
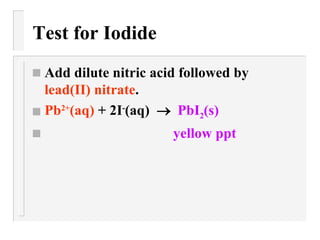
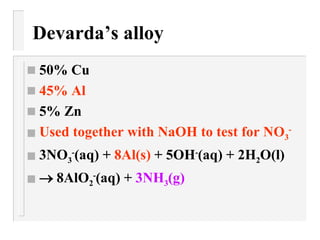
This document describes methods to identify different anions through precipitation reactions. It lists the anions that can be identified as nitrate, sulfate, iodide, and carbonate. For each anion, it provides the reagent used to cause precipitation and the color of the precipitate formed. Identification is achieved by observing the precipitate color during reactions with specific reagents.