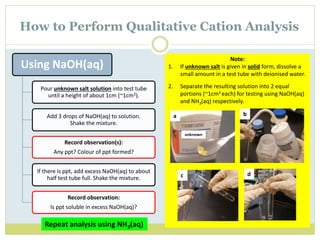
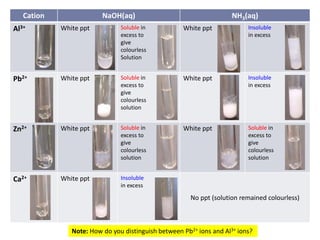
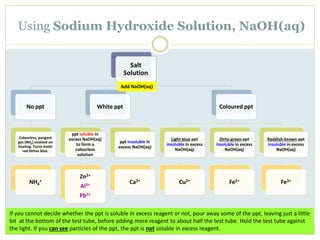
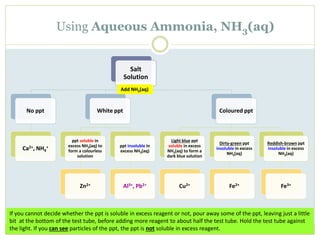
This document provides instructions for identifying cations through qualitative analysis using sodium hydroxide (NaOH) and ammonium hydroxide (NH3) solutions. Precipitates formed when salts are reacted with these reagents can indicate the present metal ions. Observations of solubility in excess reagent and reactions with other substances like hydrochloric acid help distinguish between ions. Proper technique like warming solutions gently and testing gas evolution with litmus paper is emphasized.



![Test for Ammonium Ion
Things to note:
1)Remember to wet litmus
paper before use. Can you
recall the reason?
[A moist
litmus paper allows the gas
to dissolve in water, forming
H+(aq) or OH-(aq) ions.]
2)Observe how warming is
done: Remove test tube
from Bunsen flame
periodically to prevent
contents from spurting.](https://image.slidesharecdn.com/cationqualitativeanalysis-101122003124-phpapp01/85/Cation-qualitative-analysis-8-320.jpg)
