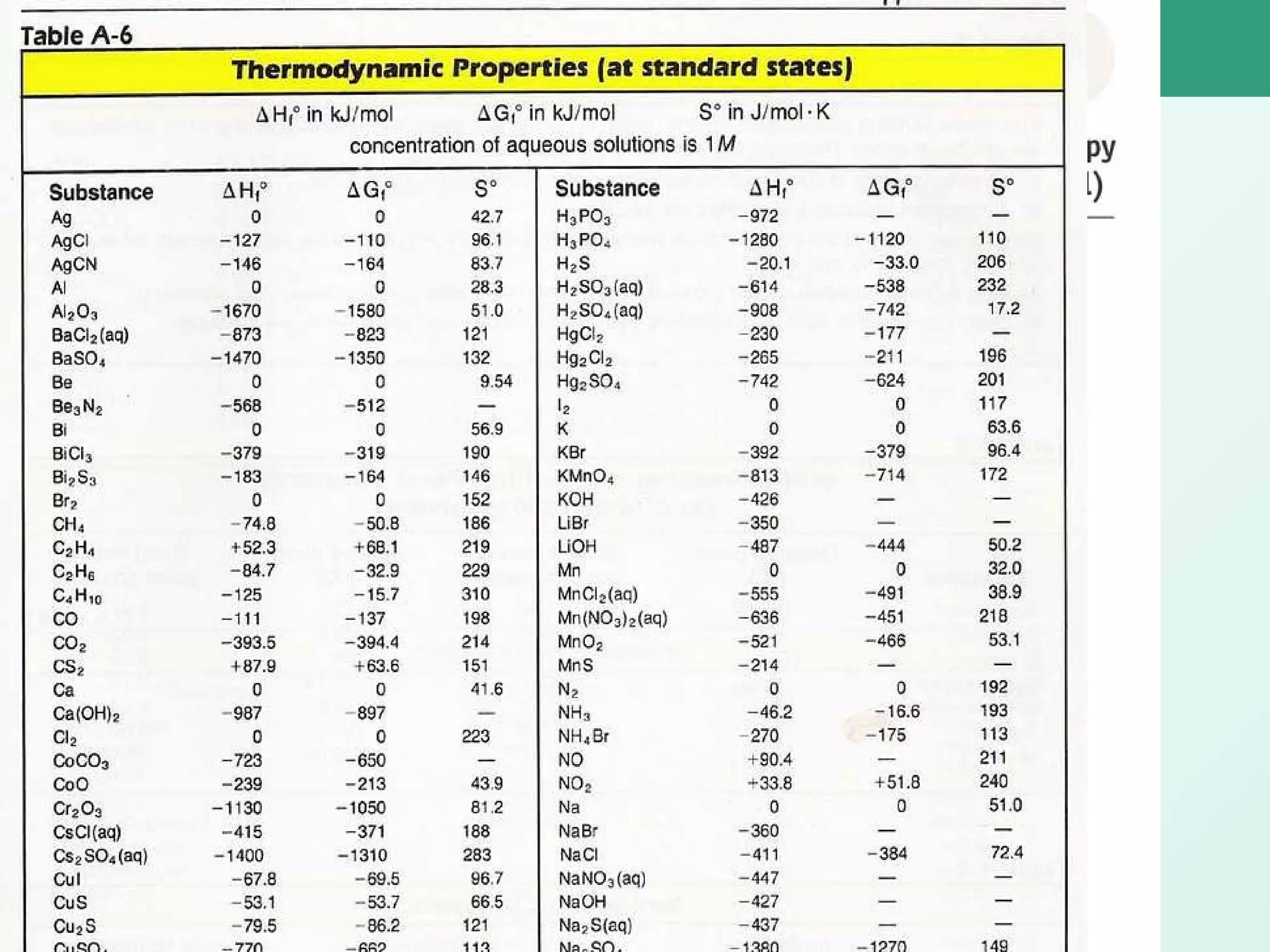
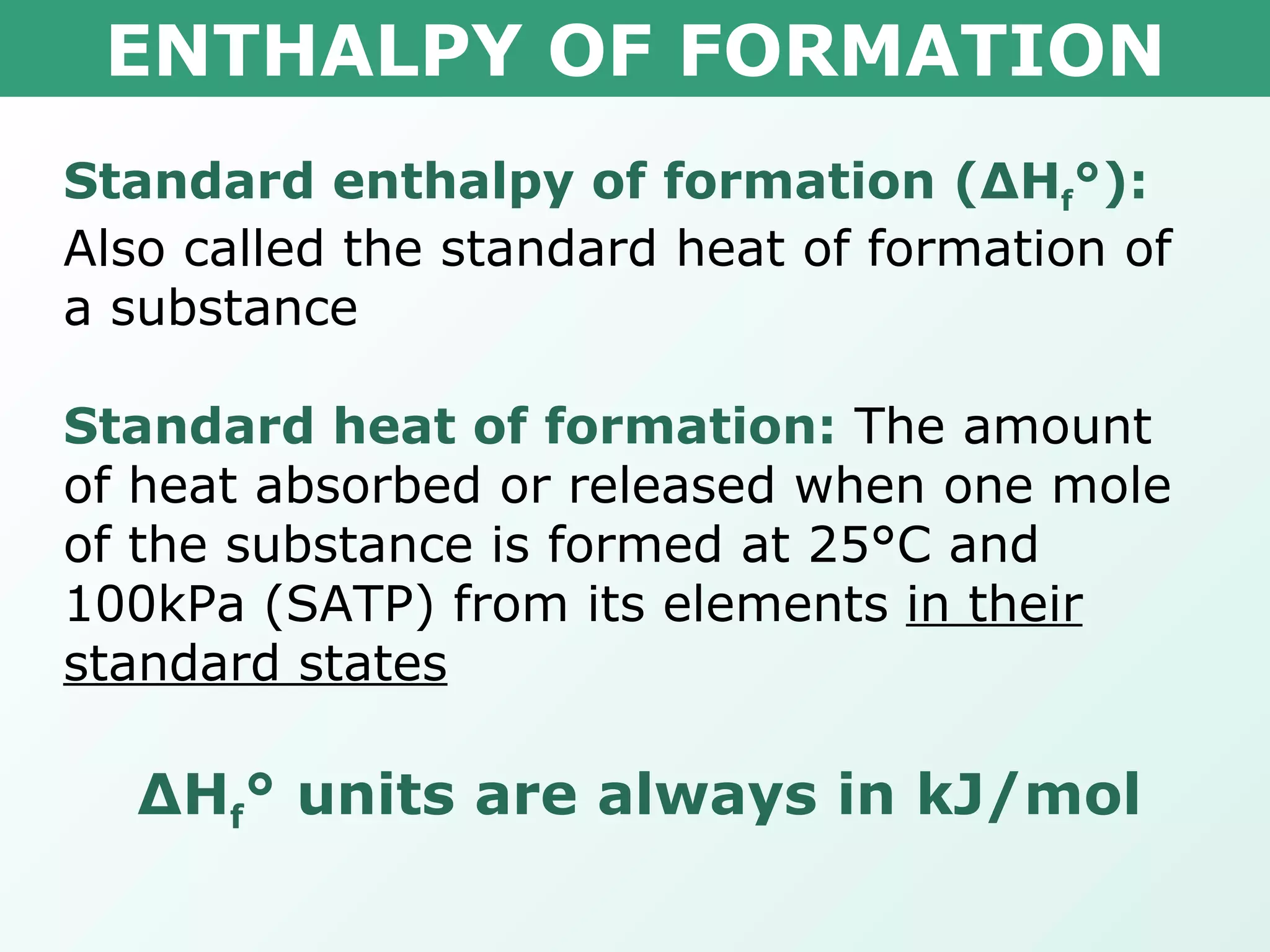
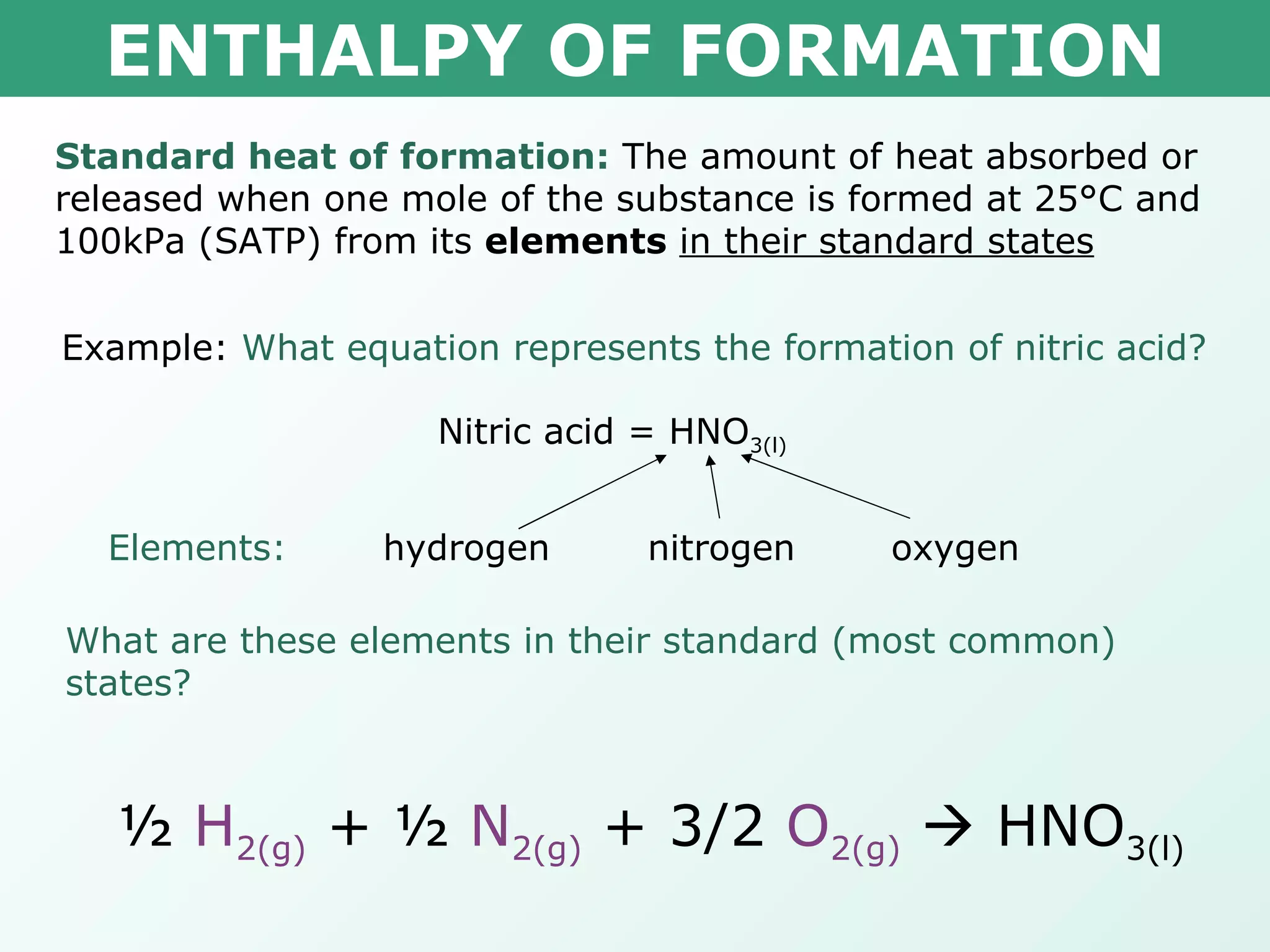
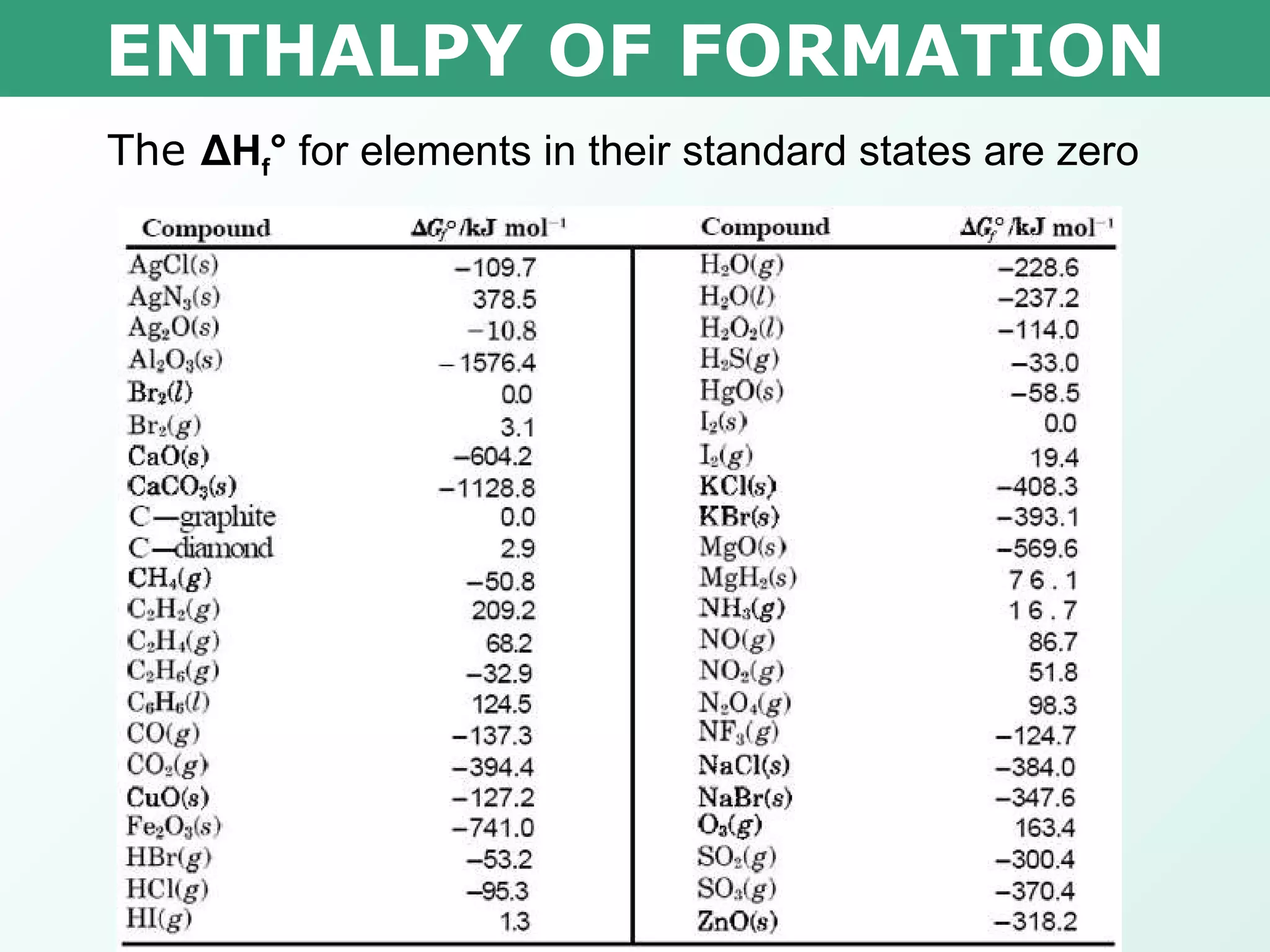
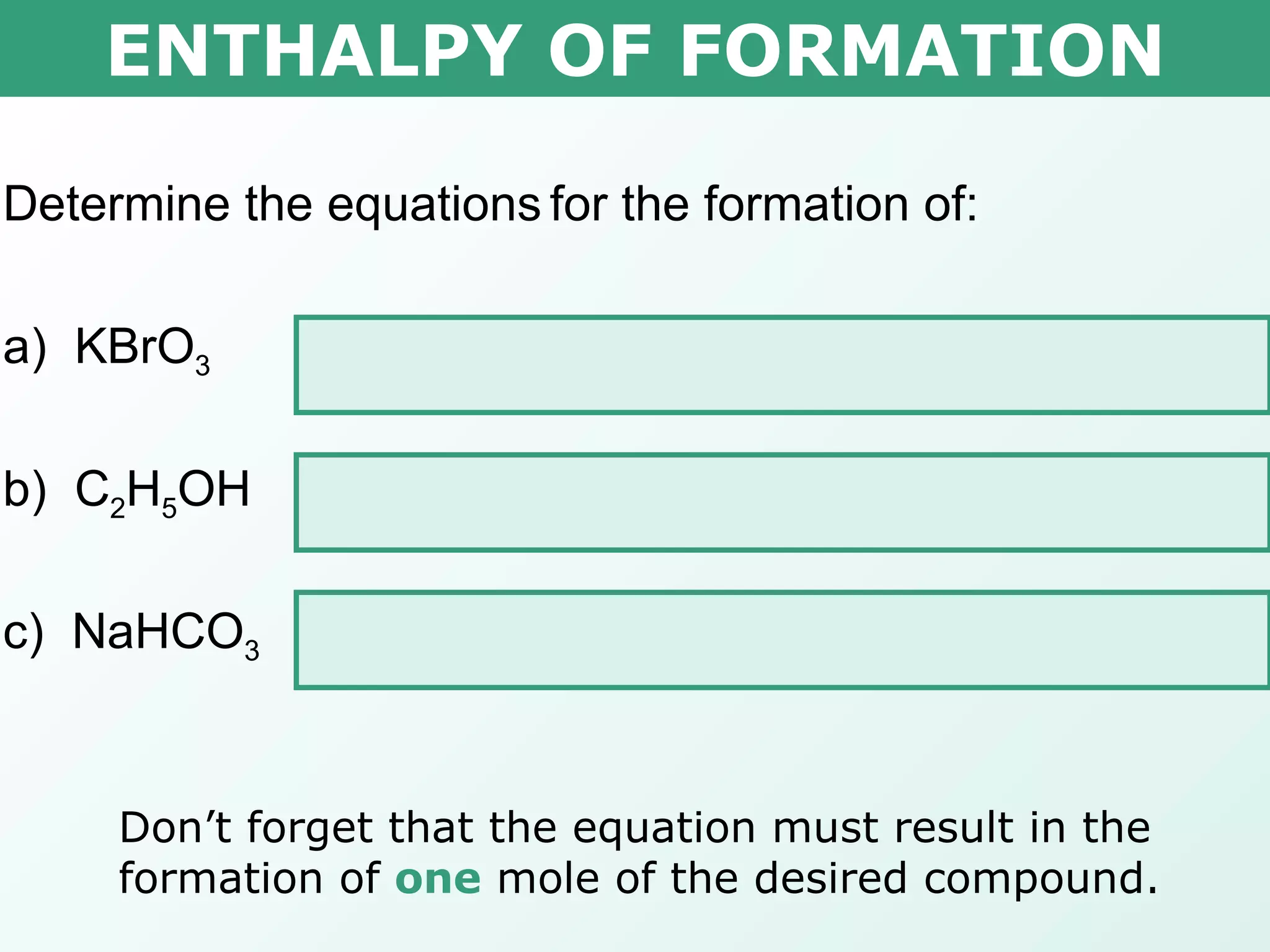
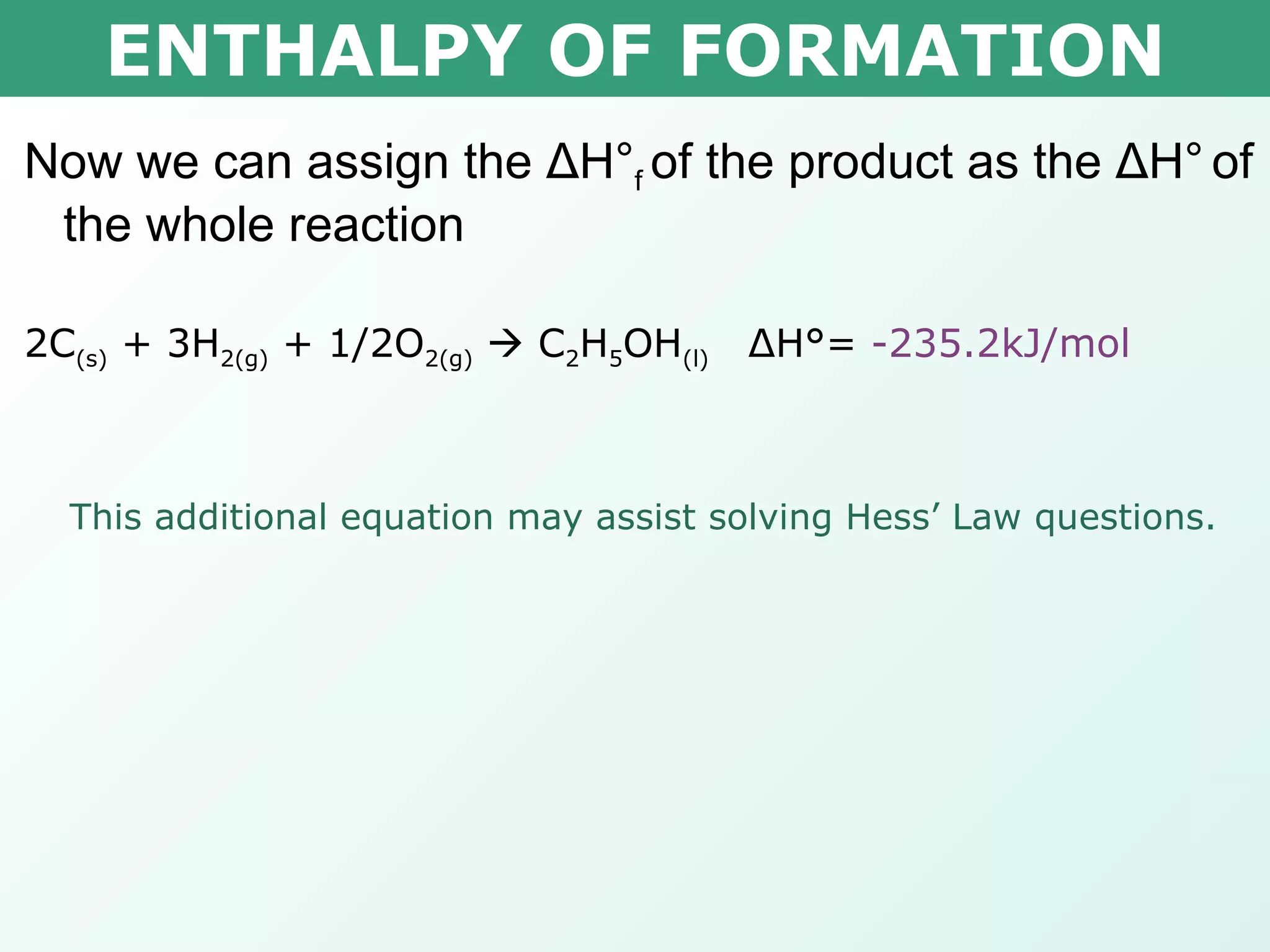
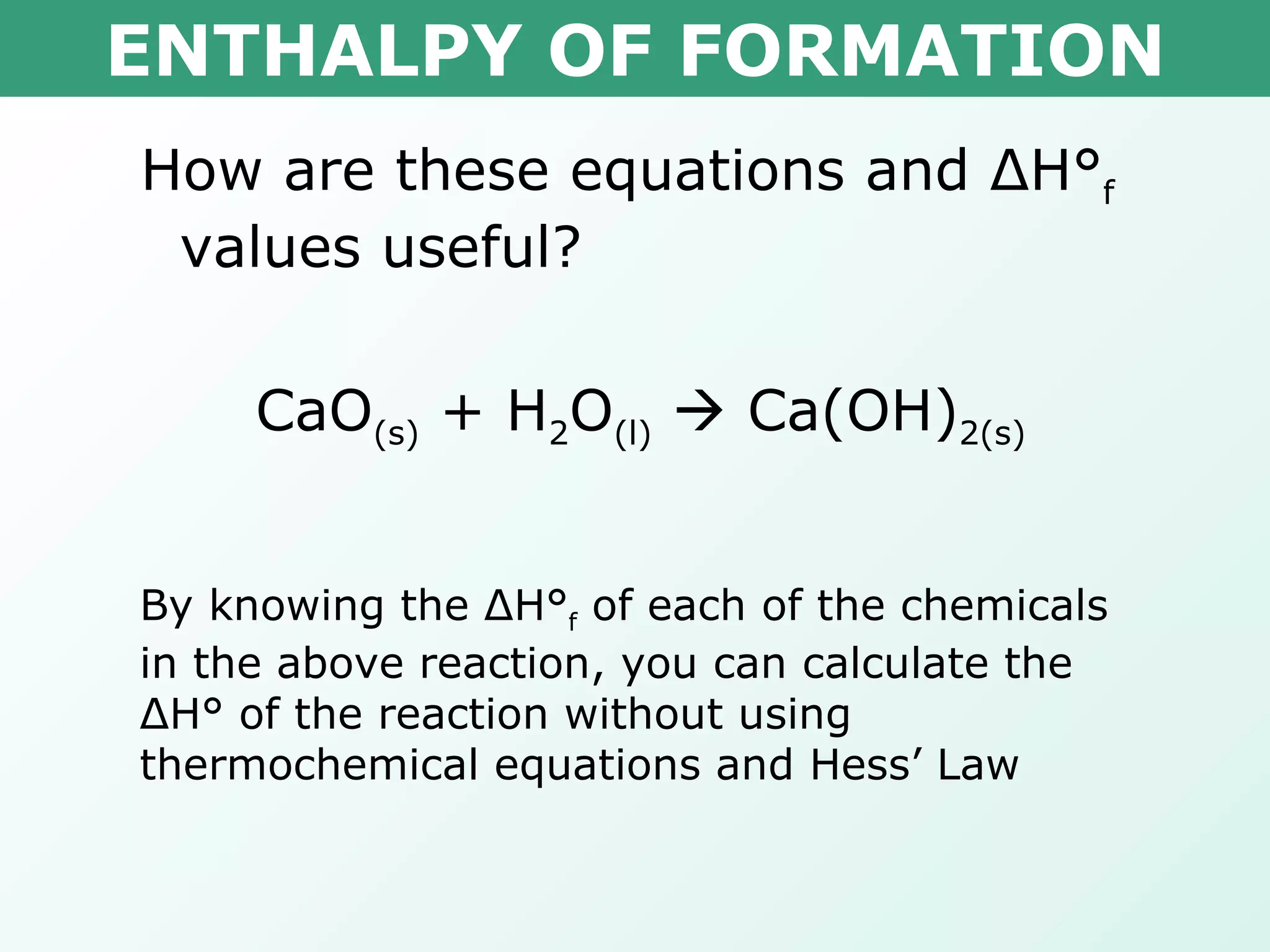
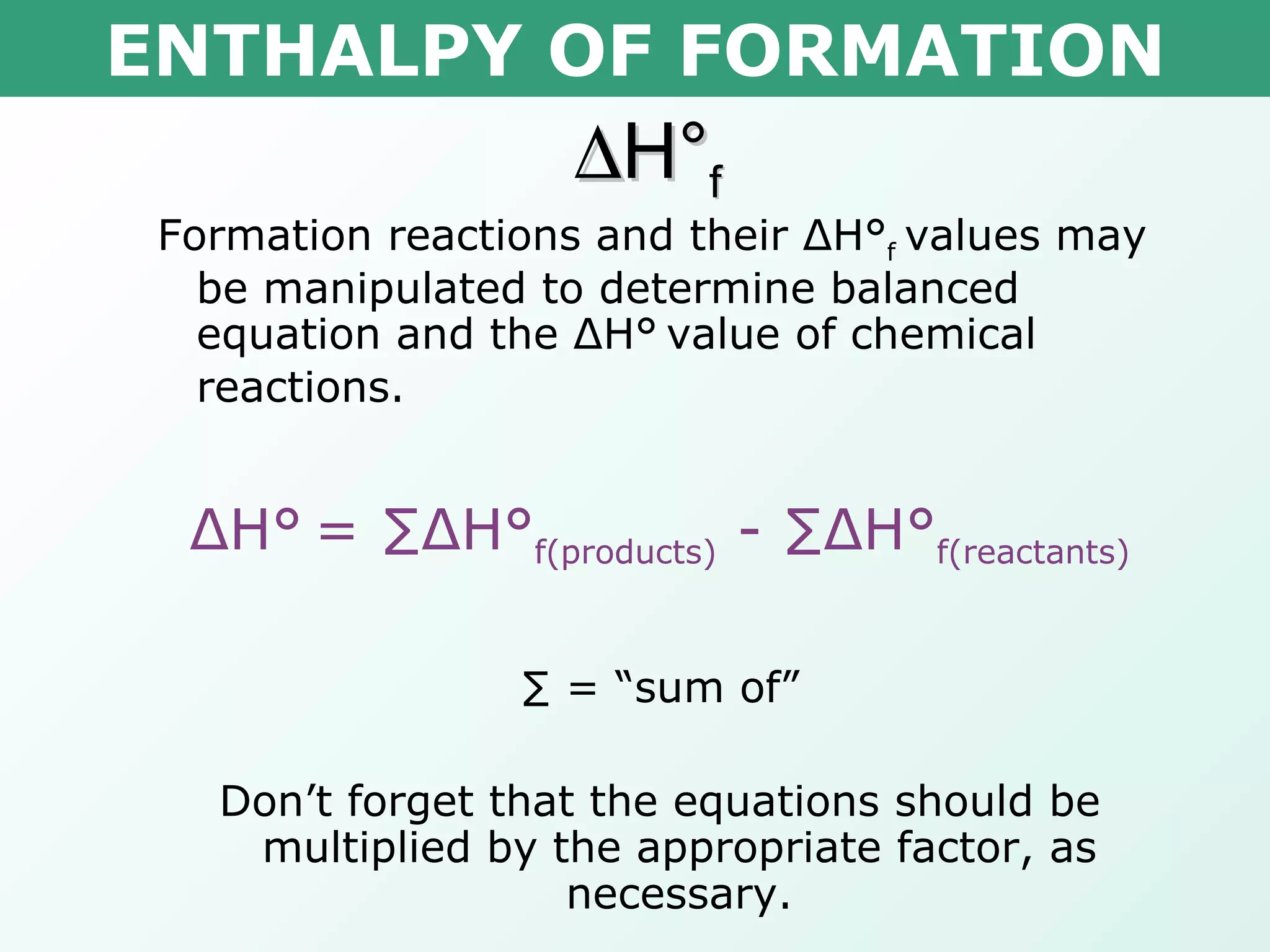
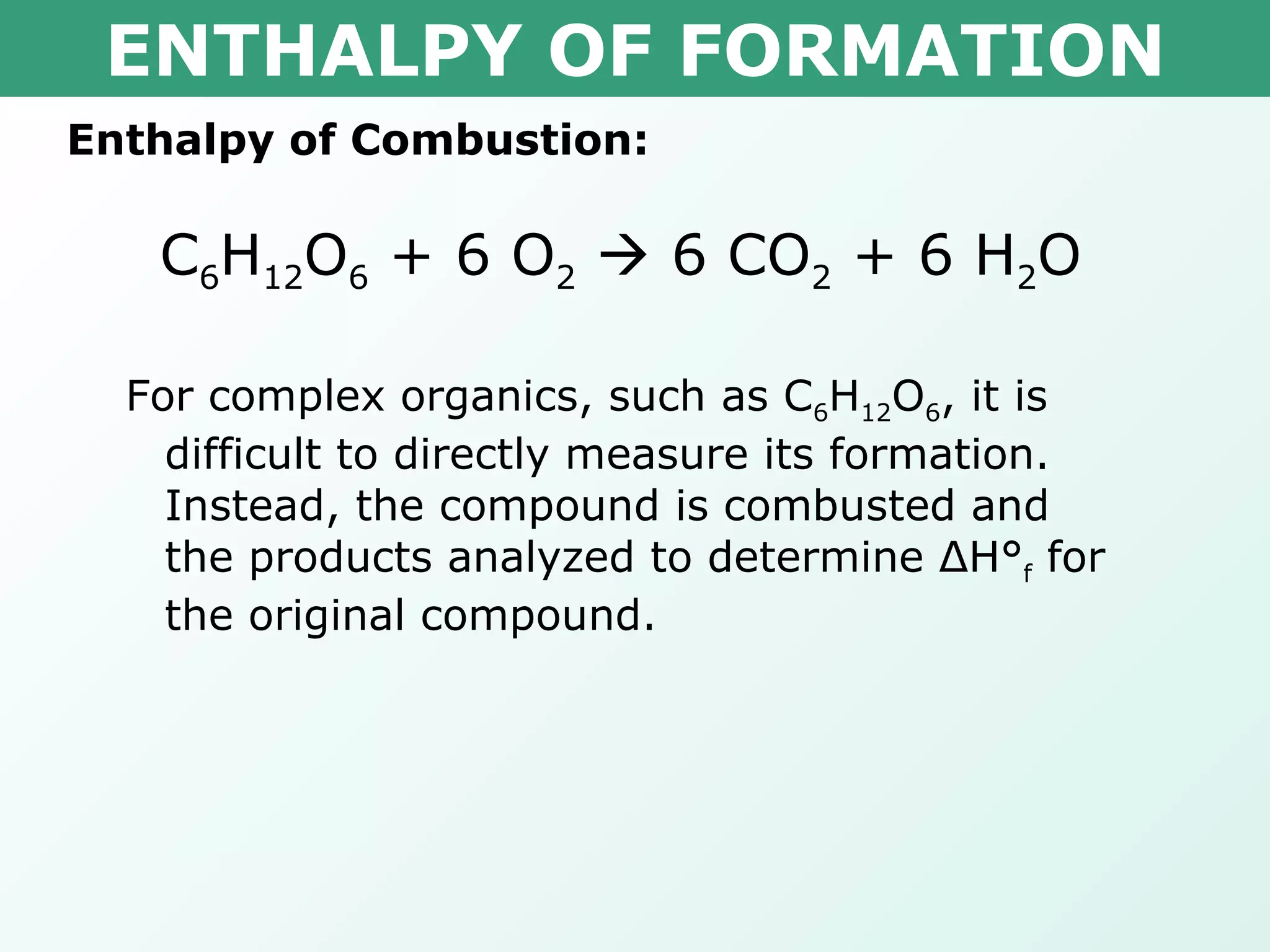
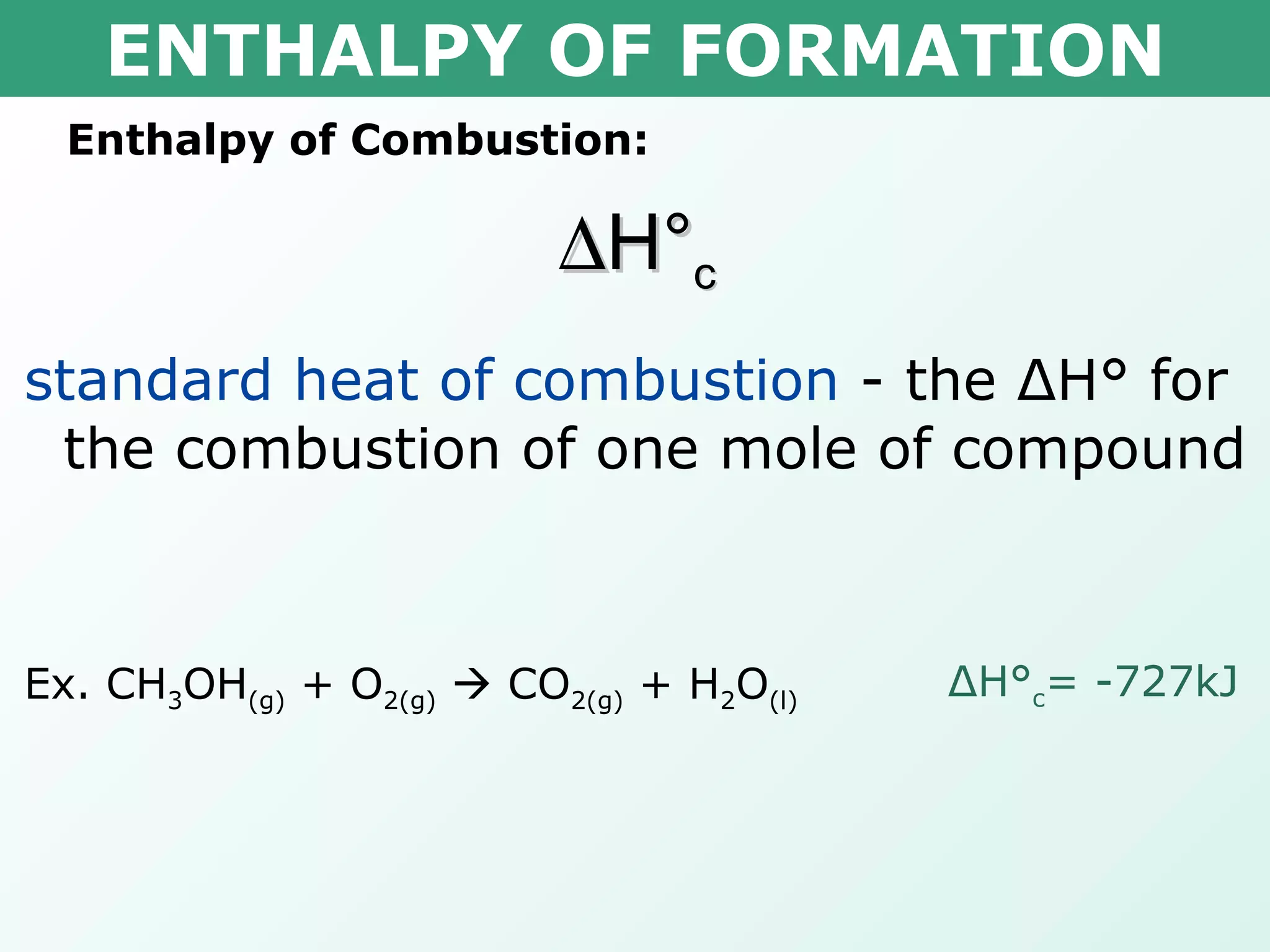
The document discusses standard enthalpy of formation (ΔHf°), which is the amount of heat absorbed or released when one mole of a substance is formed from its elements in their standard states. Examples are provided of writing balanced chemical equations for formation reactions and using ΔHf° values to calculate the enthalpy change (ΔH°) of chemical reactions. The standard heat of combustion (ΔHc°) is also introduced, which is the enthalpy change when a substance undergoes complete combustion.

![ENTHALPY OF FORMATION Using Δ H° f values, calculate the Δ H° of combustion of one mole of ethanol to produce carbon dioxide gas and liquid water. C 2 H 5 OH (l) + O 2(g) CO 2(g) + H 2 O (l) 3 2 3 -235.2kJ/mol 0.0000kJ/mol -393.5kJ/mol -285.8kJ/mol Δ H° = ∑ Δ H° f(products) - ∑ Δ H° f(reactants) = [(2 mol x -393.5kJ/mol) + (3 mol x -285.8kJ/mol)] – [(1 mol x -235.2kJ/mol) + (3 mol x 0.0000kJ/mol)] = -1644.4kJ – (-235.2kJ) = -1409.2kJ Since this equation already combusts 1 mole of ethanol, then the final answer is: Therefore the heat of combustion is -1409kJ/mol of ethanol](https://image.slidesharecdn.com/tang03-enthalpyofformationandcombustion-120209095955-phpapp01/75/Tang-03-enthalpy-of-formation-and-combustion-9-2048.jpg)
![ENTHALPY OF FORMATION Using Δ H° f values, calculate the Δ H° for the following reaction: NaOH (s) + HCl (g) NaCl (s) + H 2 O (l) -425.6kJ/mol -92.30kJ/mol -411.2kJ/mol -285.8kJ/mol Δ H° = ∑ Δ H° f(products) - ∑ Δ H° f(reactants) = [(1 mol x –411.2kJ/mol) + (1 mol x -285.8kJ/mol)] – [(1 mol x -425.6kJ/mol) + (1 mol x -92.30kJ/mol)] = -697.0kJ – (-517.9kJ) = -179.1kJ Therefore the heat of the reaction is -179.1kJ](https://image.slidesharecdn.com/tang03-enthalpyofformationandcombustion-120209095955-phpapp01/75/Tang-03-enthalpy-of-formation-and-combustion-10-2048.jpg)
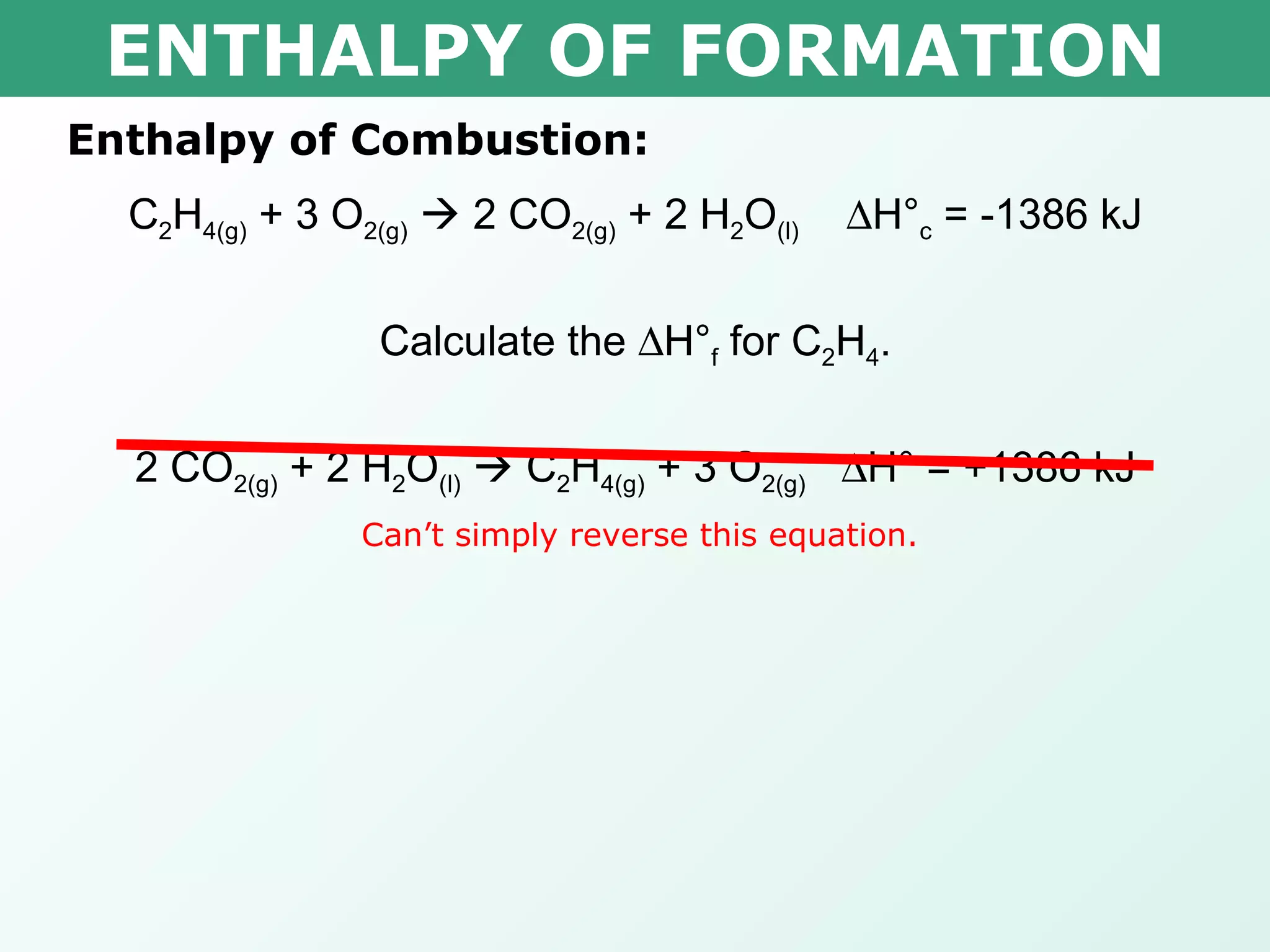
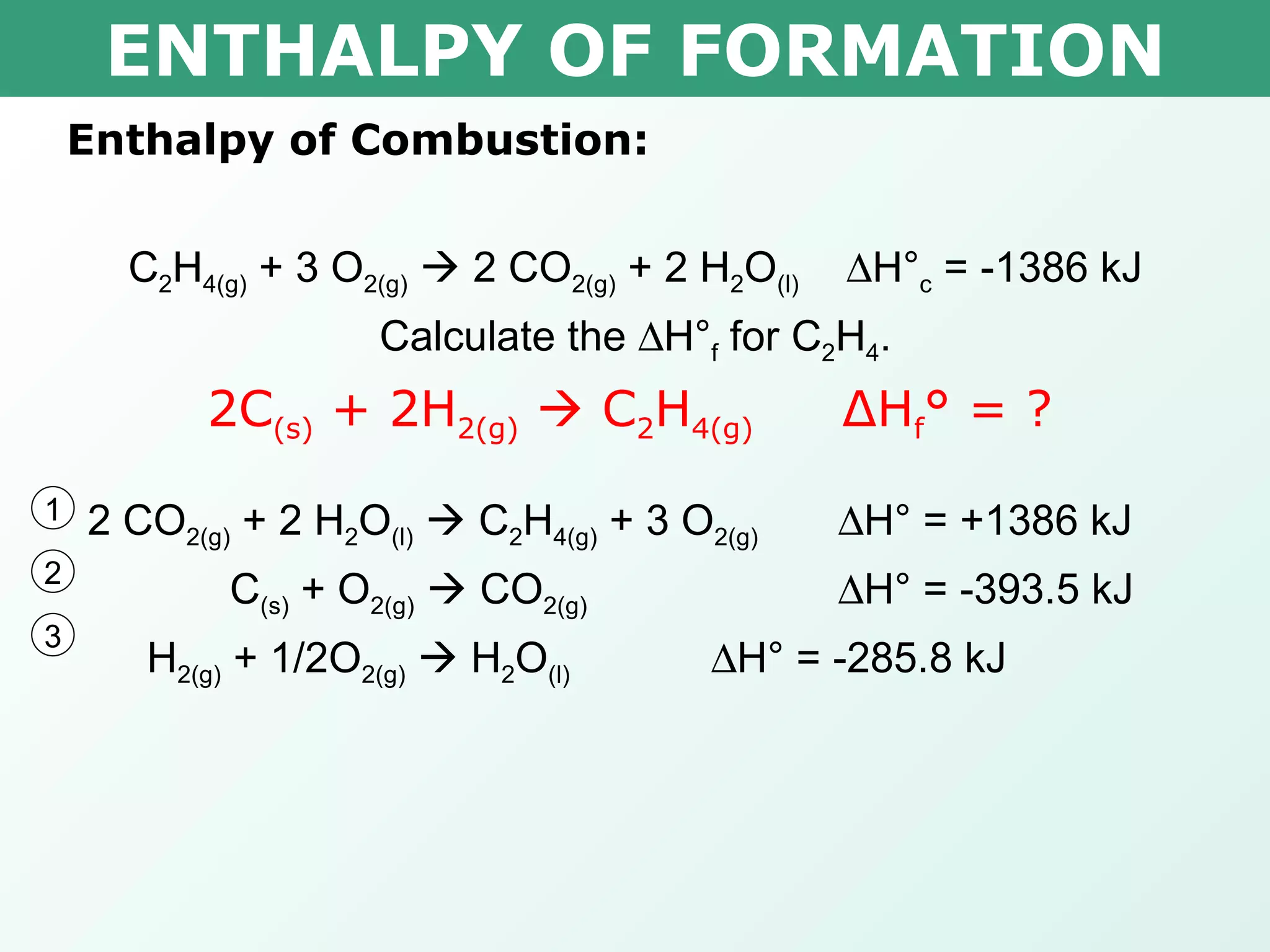
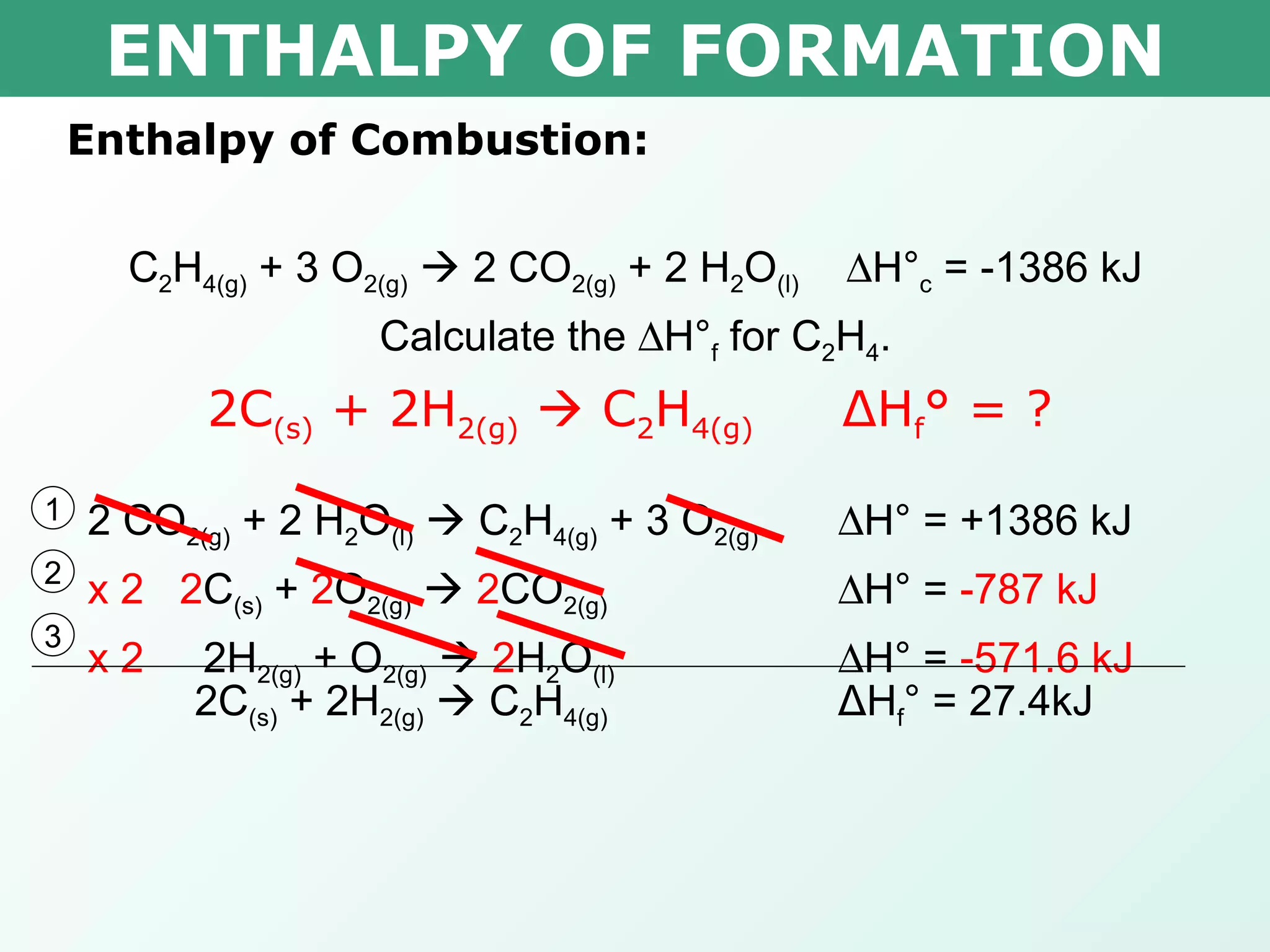
![ENTHALPY OF FORMATION x 0.000kJ -393.5kJ -285.8kJ Δ H° = ∑ Δ H° f(products) - ∑ Δ H° f(reactants) -1386kJ= [(2 mol x -393.5kJ/mol) + (2 mol x -285.8kJ/mol)] – [(1 mol x x ) + (3 mol x 0.000kJ/mol)] -1386kJ= -1358.6kJ – x x = 27.4kJ Therefore the heat of formation is 27.4kJ/mol Calculate the H° f for C 2 H 4 . C 2 H 4(g) + 3 O 2(g) 2 CO 2(g) + 2 H 2 O (l) H° c = -1386 kJ](https://image.slidesharecdn.com/tang03-enthalpyofformationandcombustion-120209095955-phpapp01/75/Tang-03-enthalpy-of-formation-and-combustion-15-2048.jpg)