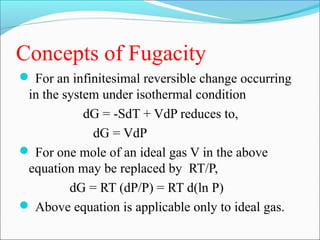
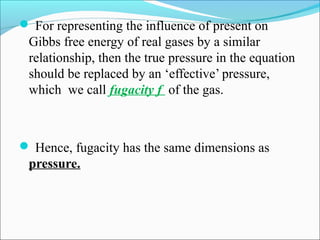
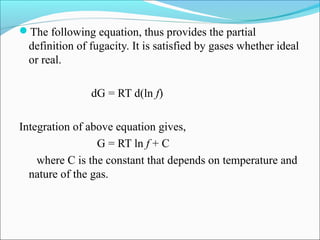
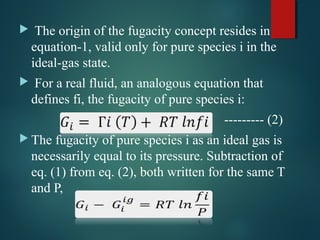
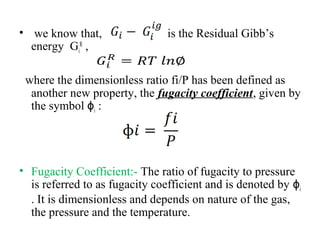
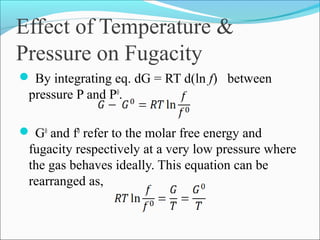
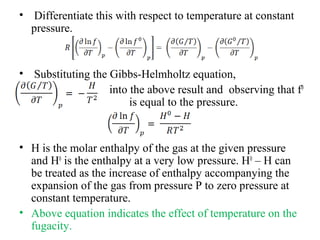
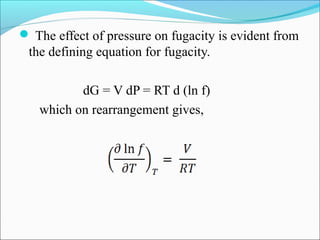
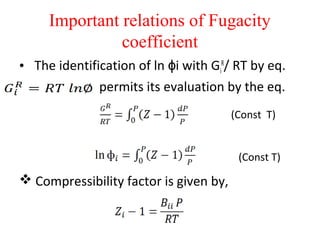
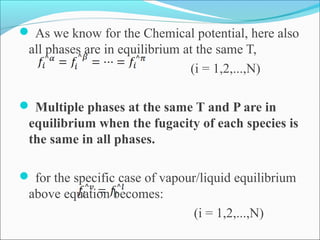
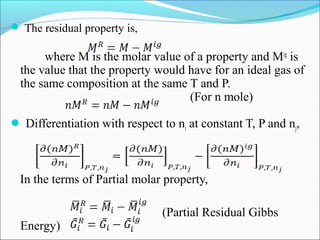
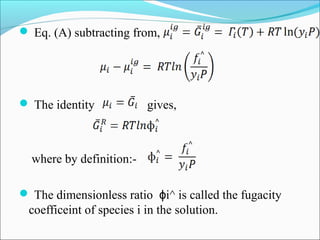
This document discusses the concept of fugacity and its coefficient in chemical engineering thermodynamics, focusing on their roles in representing real gas behavior and phase equilibrium. It introduces key equations and relationships, highlighting the effects of temperature and pressure on fugacity, as well as the parallels between fugacity in pure species and species in solution. The content is structured into sections covering the definition, properties, and applications of fugacity and its coefficient in chemical systems.