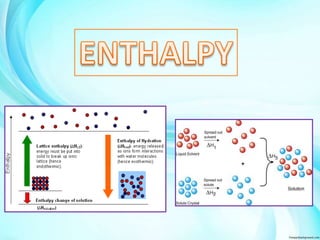
The document discusses various types of enthalpy changes including:
- Standard enthalpy of reaction is the enthalpy change when reactants and products are in their standard states.
- Enthalpy of fusion/melting is the energy required to melt 1 mole of a solid.
- Enthalpy of vaporization is the energy required to vaporize 1 mole of a liquid.
- Enthalpy of sublimation is the energy change of a solid transforming directly to a gas.
- Standard enthalpy of formation is the energy change to form 1 mole of a compound from its elements.
- Examples are provided to illustrate the different types of enthalpy changes.