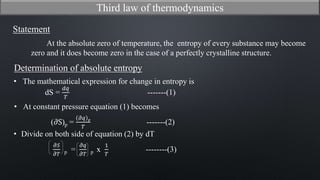
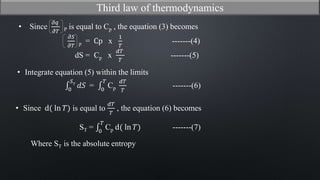
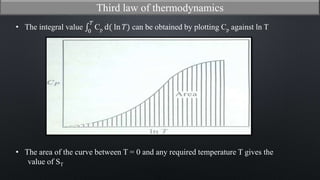
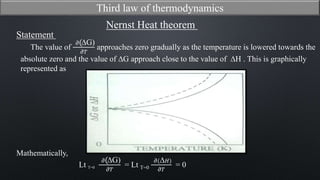
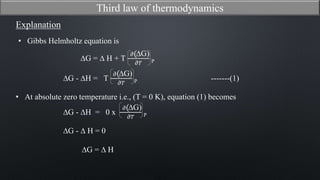
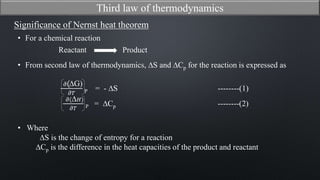
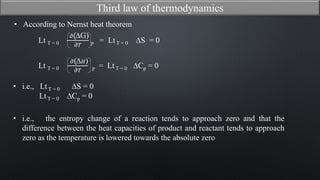
This document discusses the third law of thermodynamics. It states that the entropy of a perfectly crystalline substance is zero at absolute zero temperature. The mathematical expressions for determining absolute entropy are provided. The document also discusses Nernst's heat theorem, which states that the change in Gibbs free energy of a reaction approaches the change in enthalpy as temperature approaches absolute zero. Exceptions to the third law for certain gases with non-ordered crystal structures are also noted.