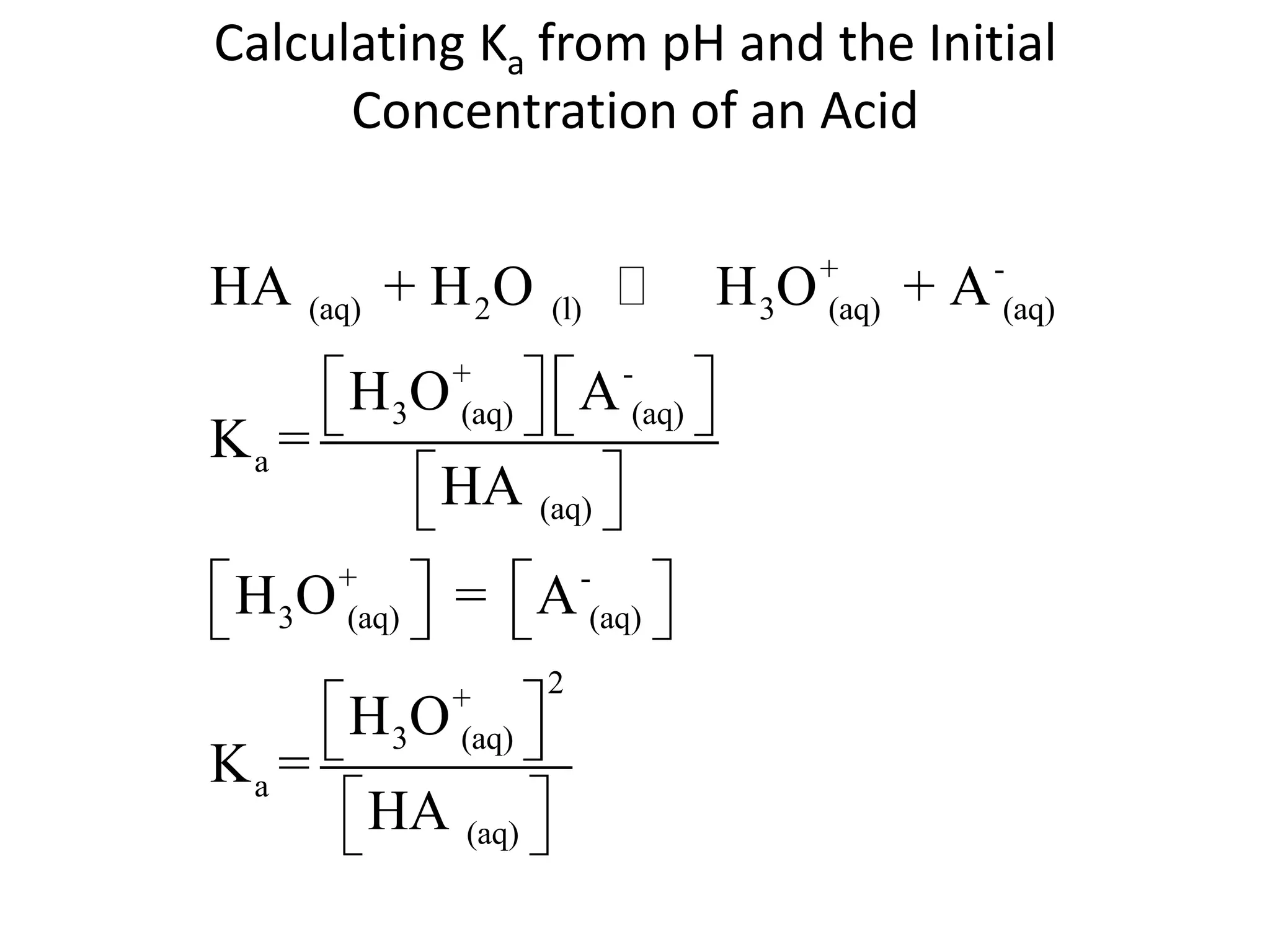
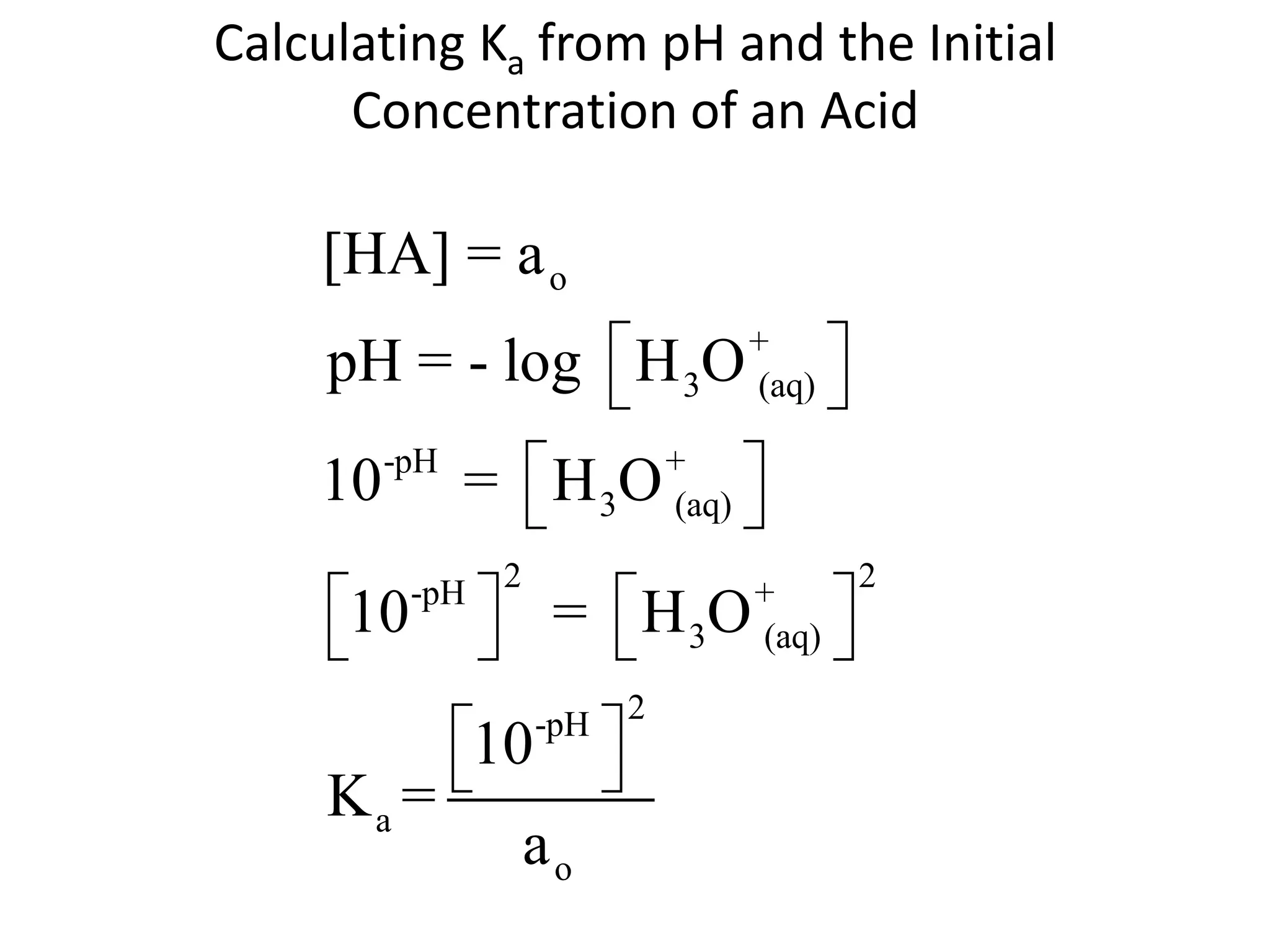
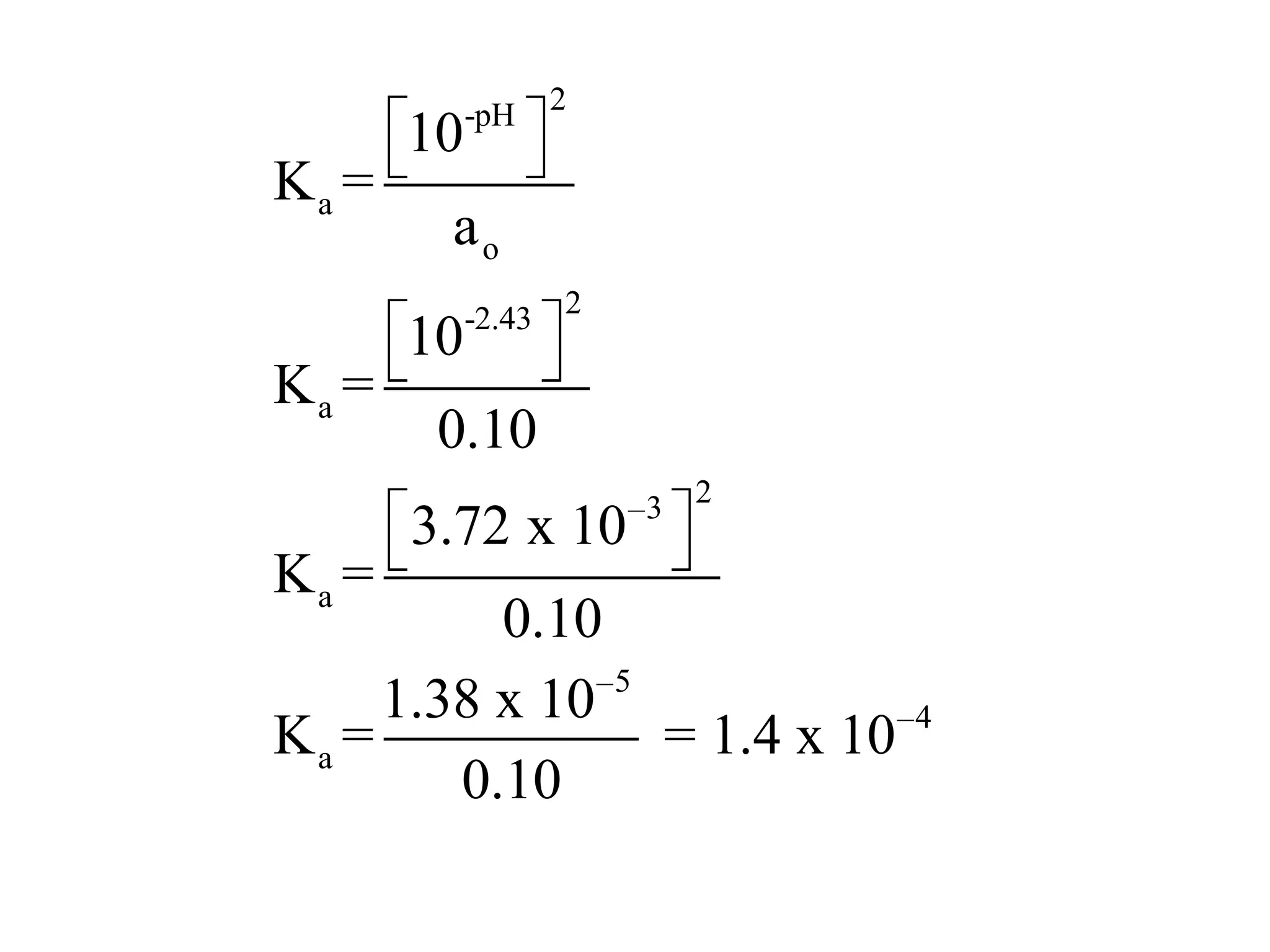
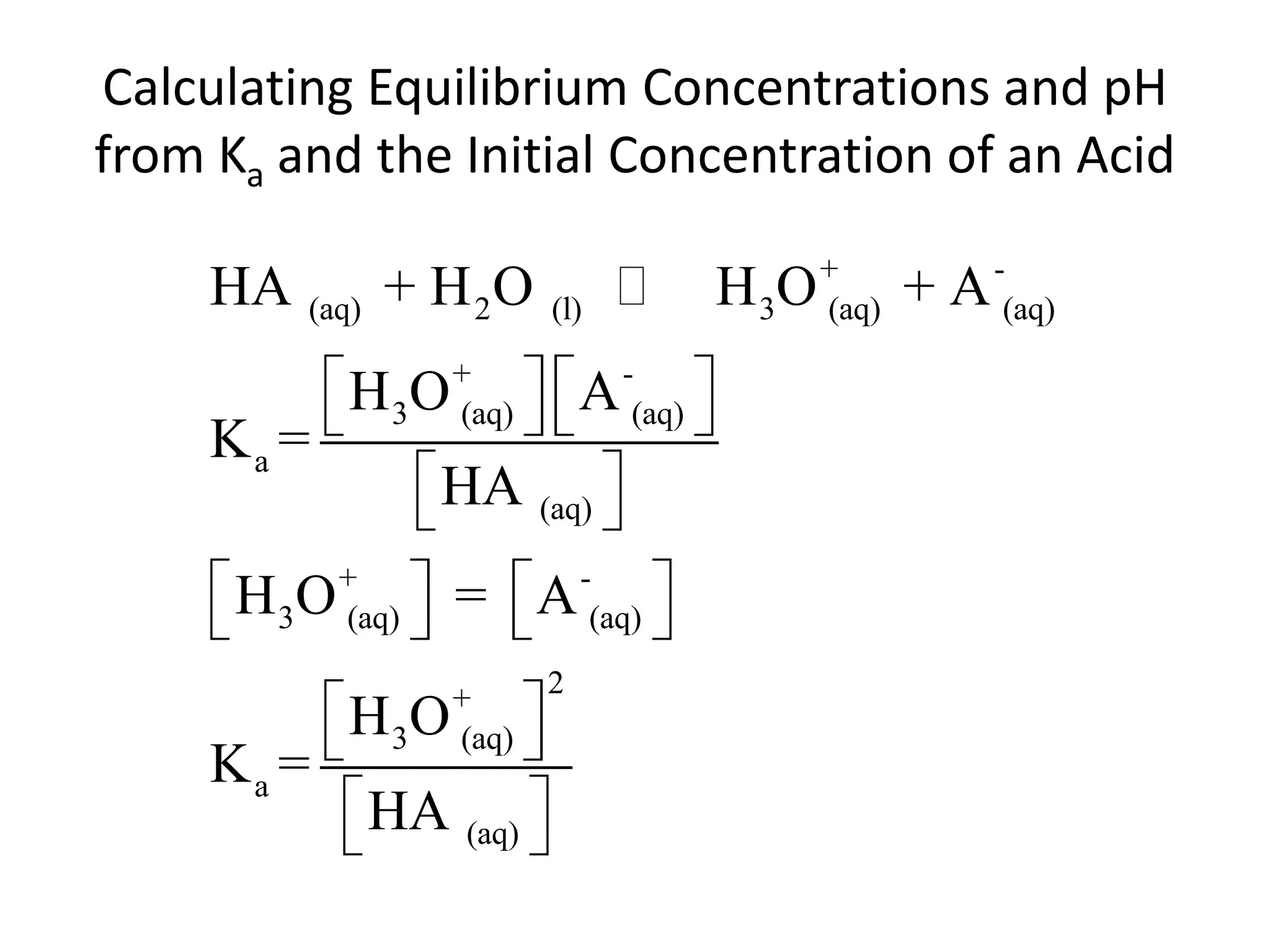
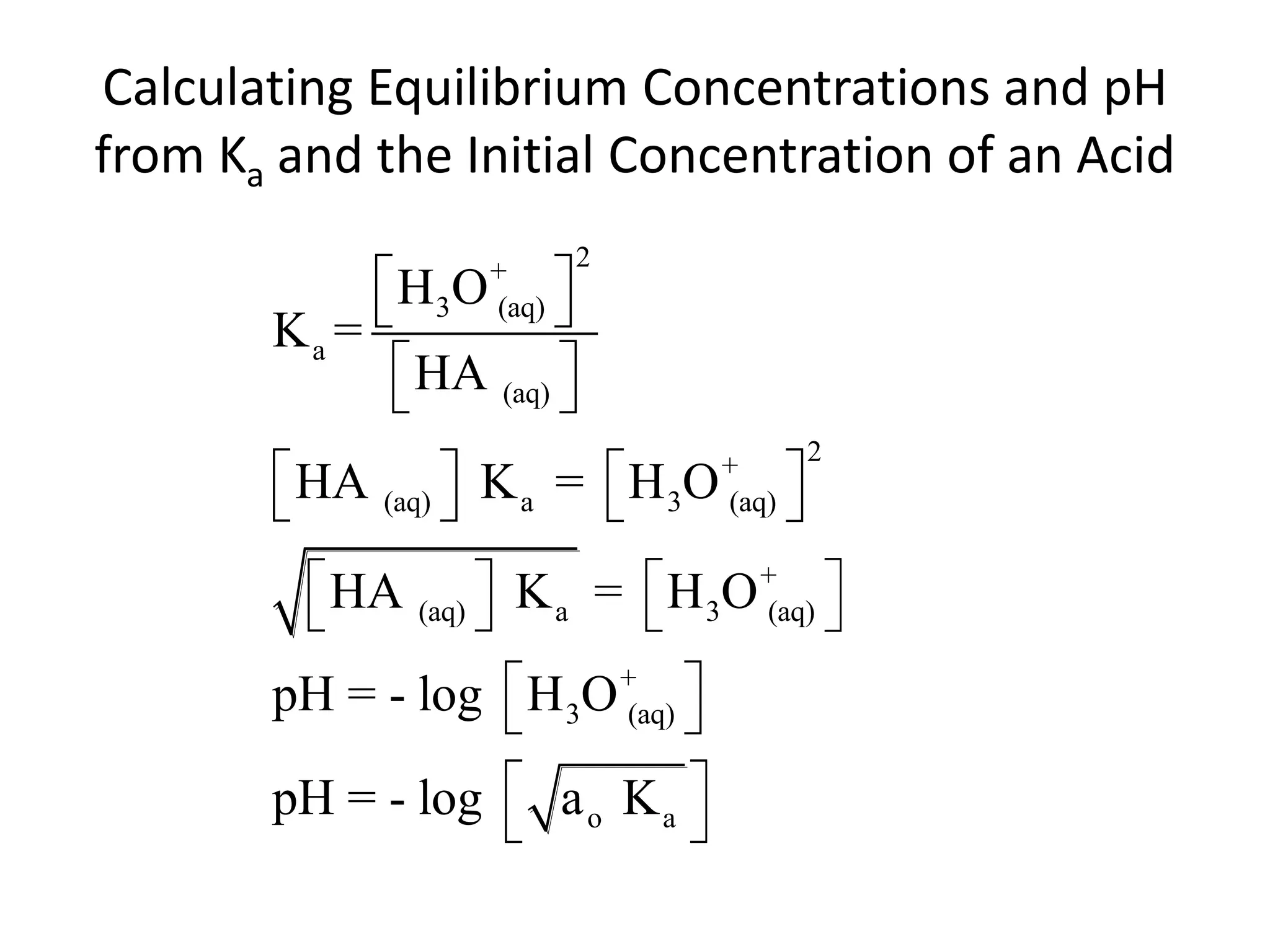
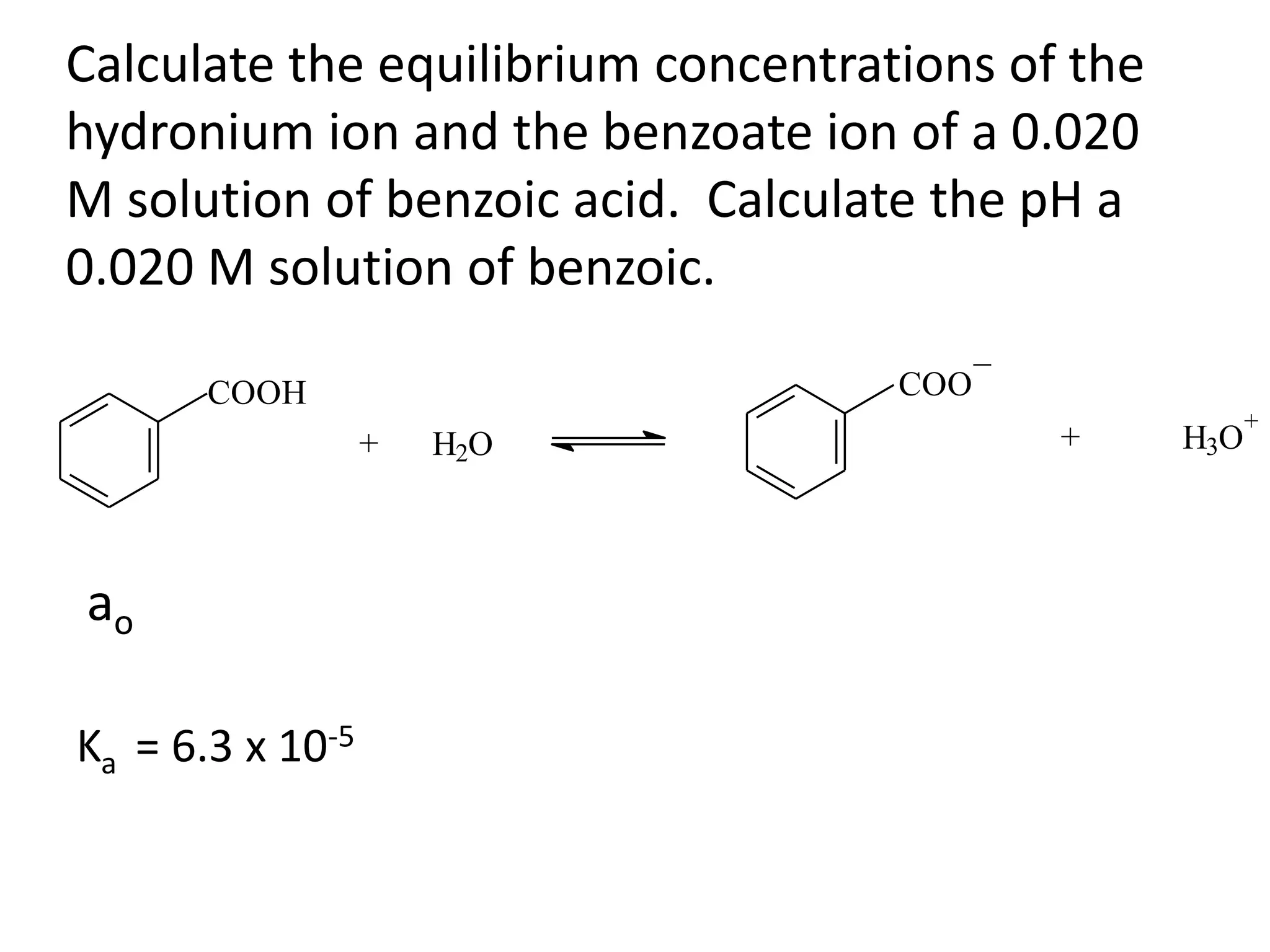
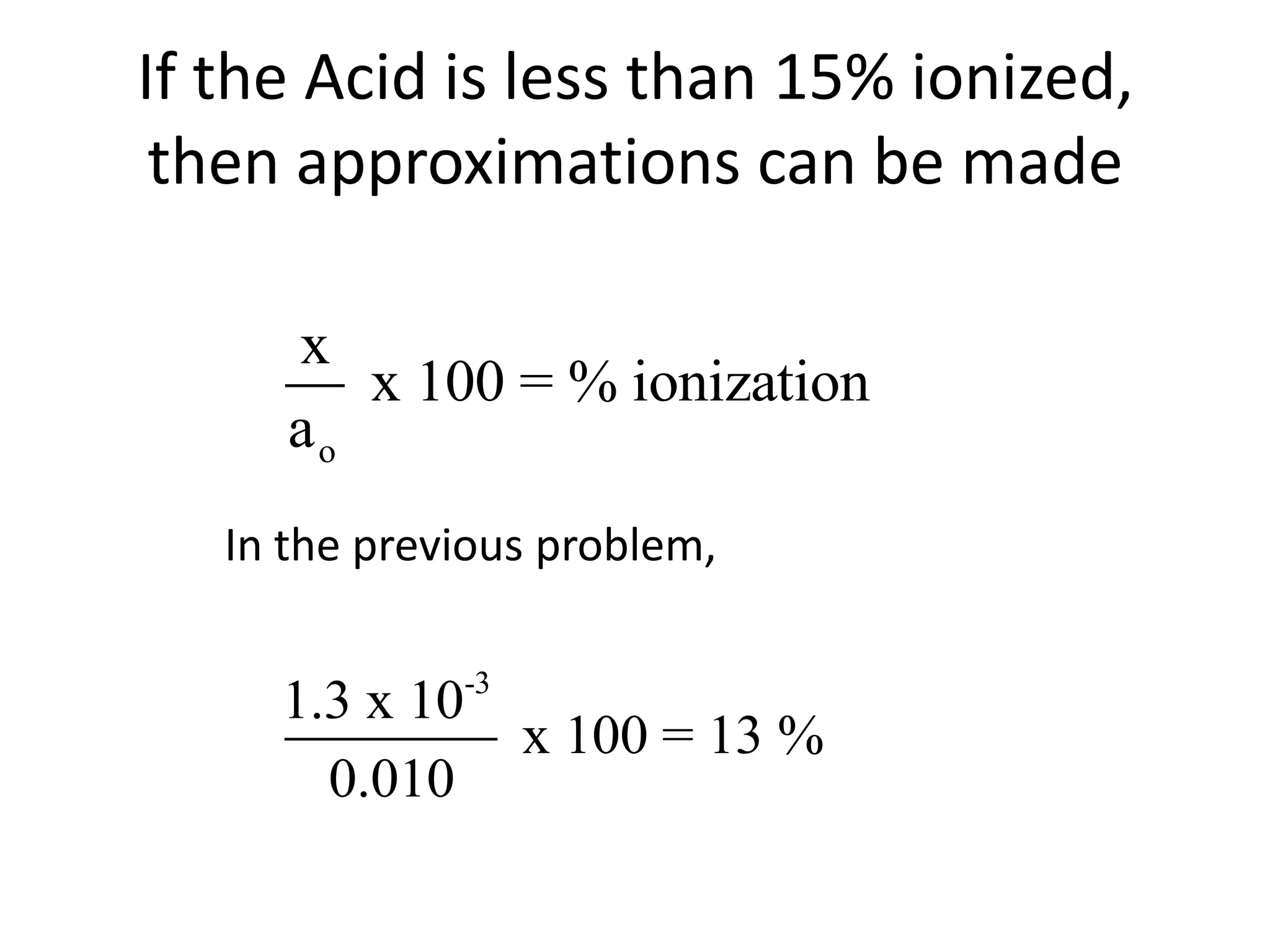
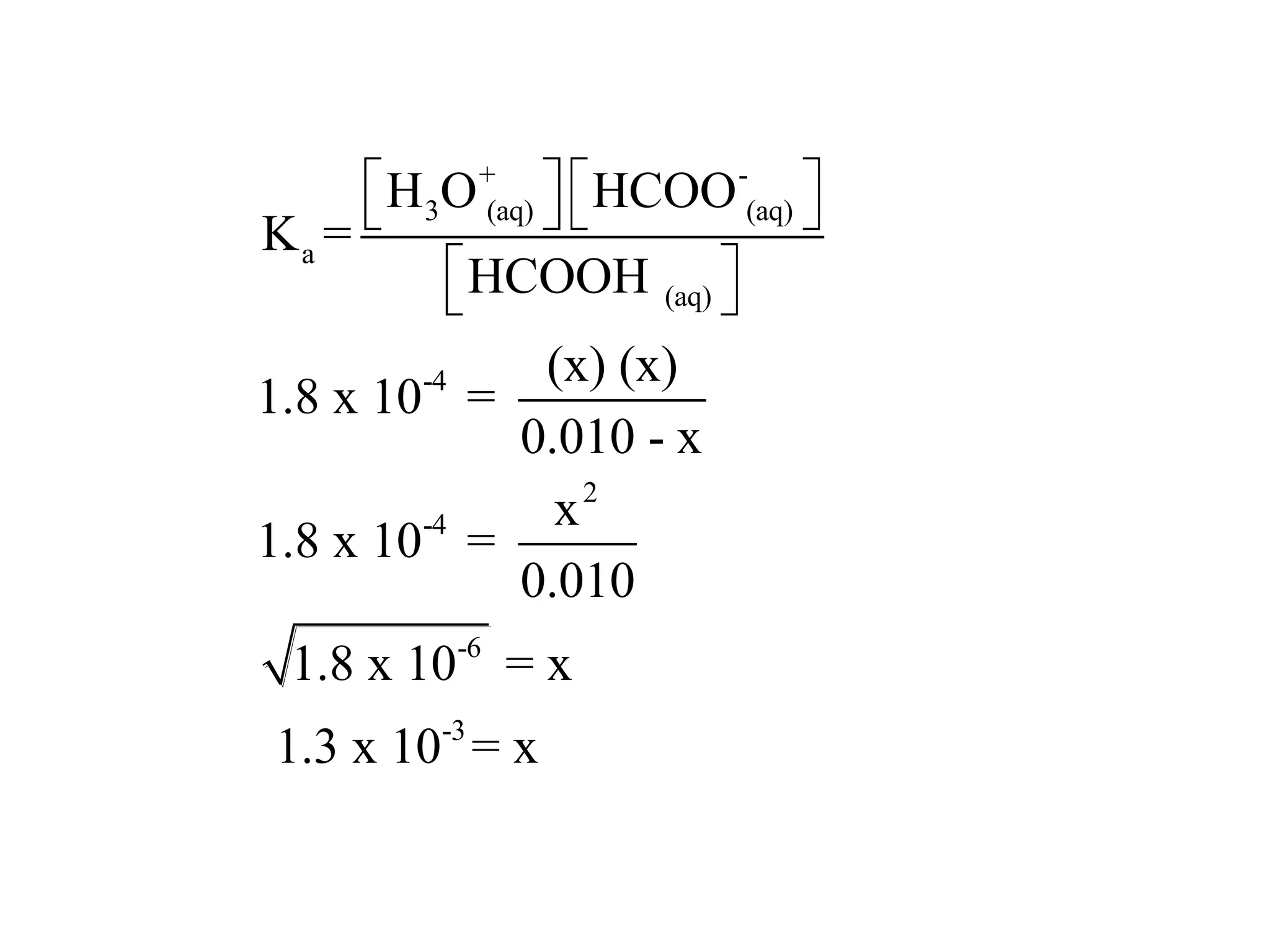
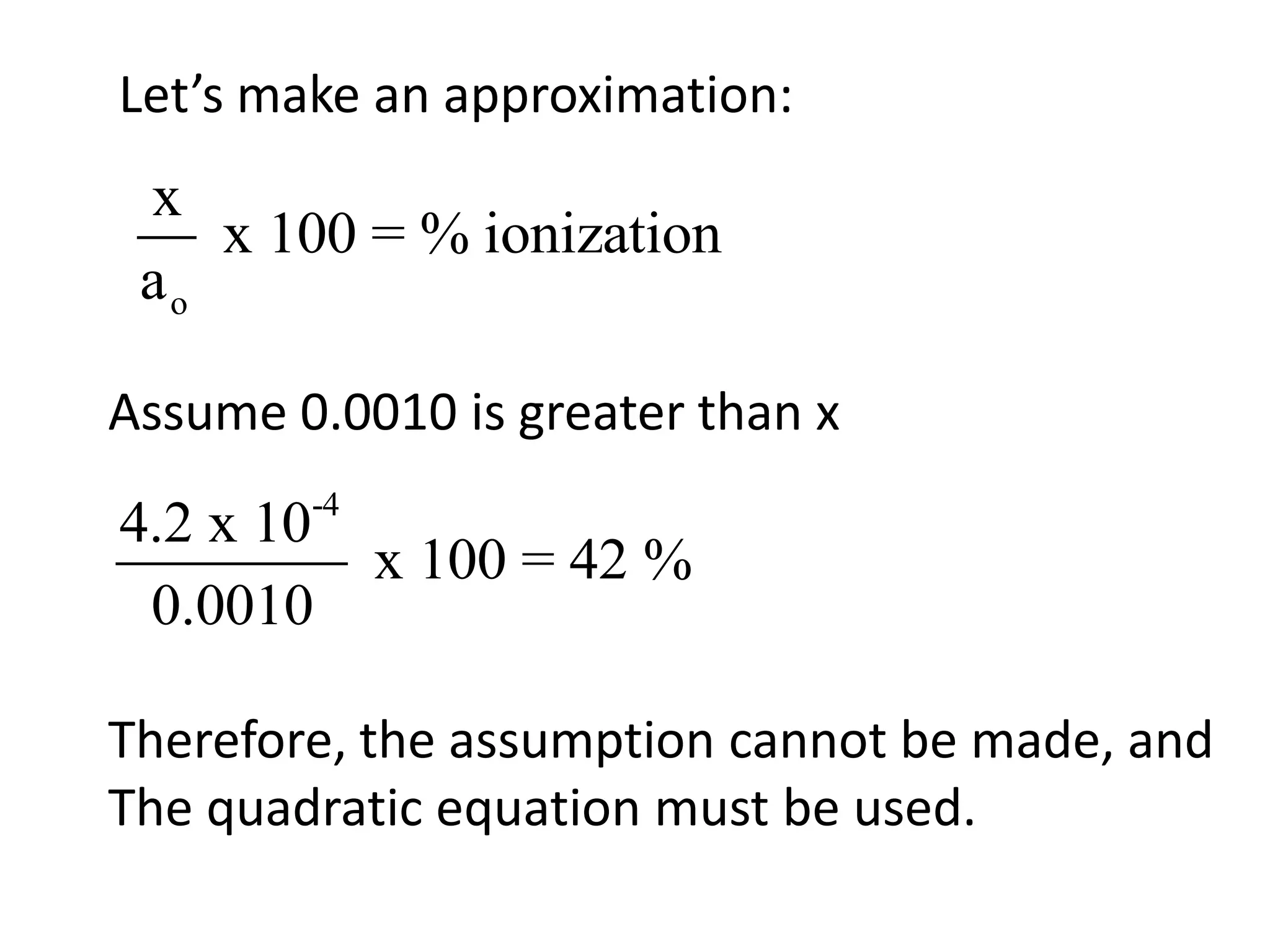
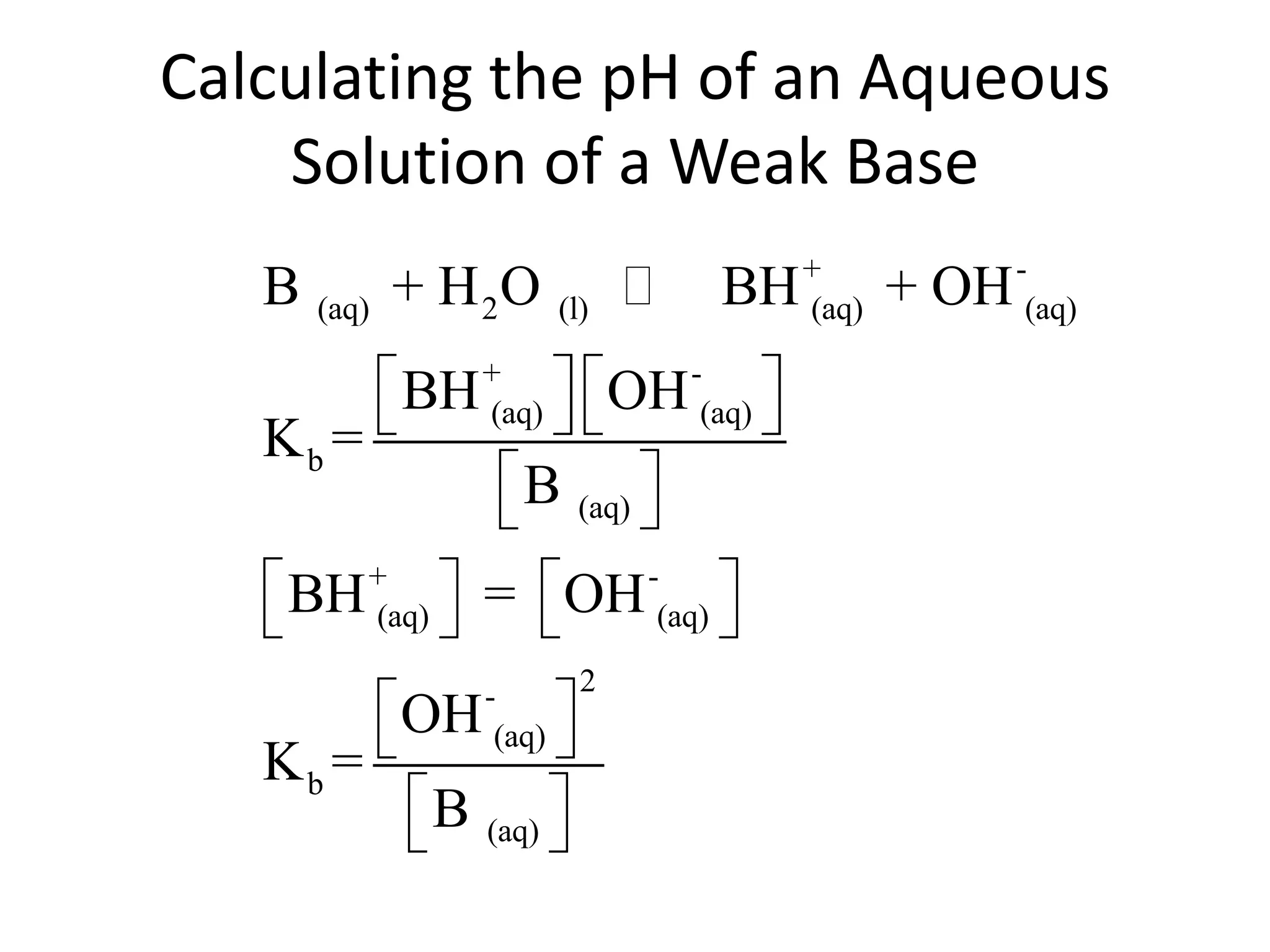
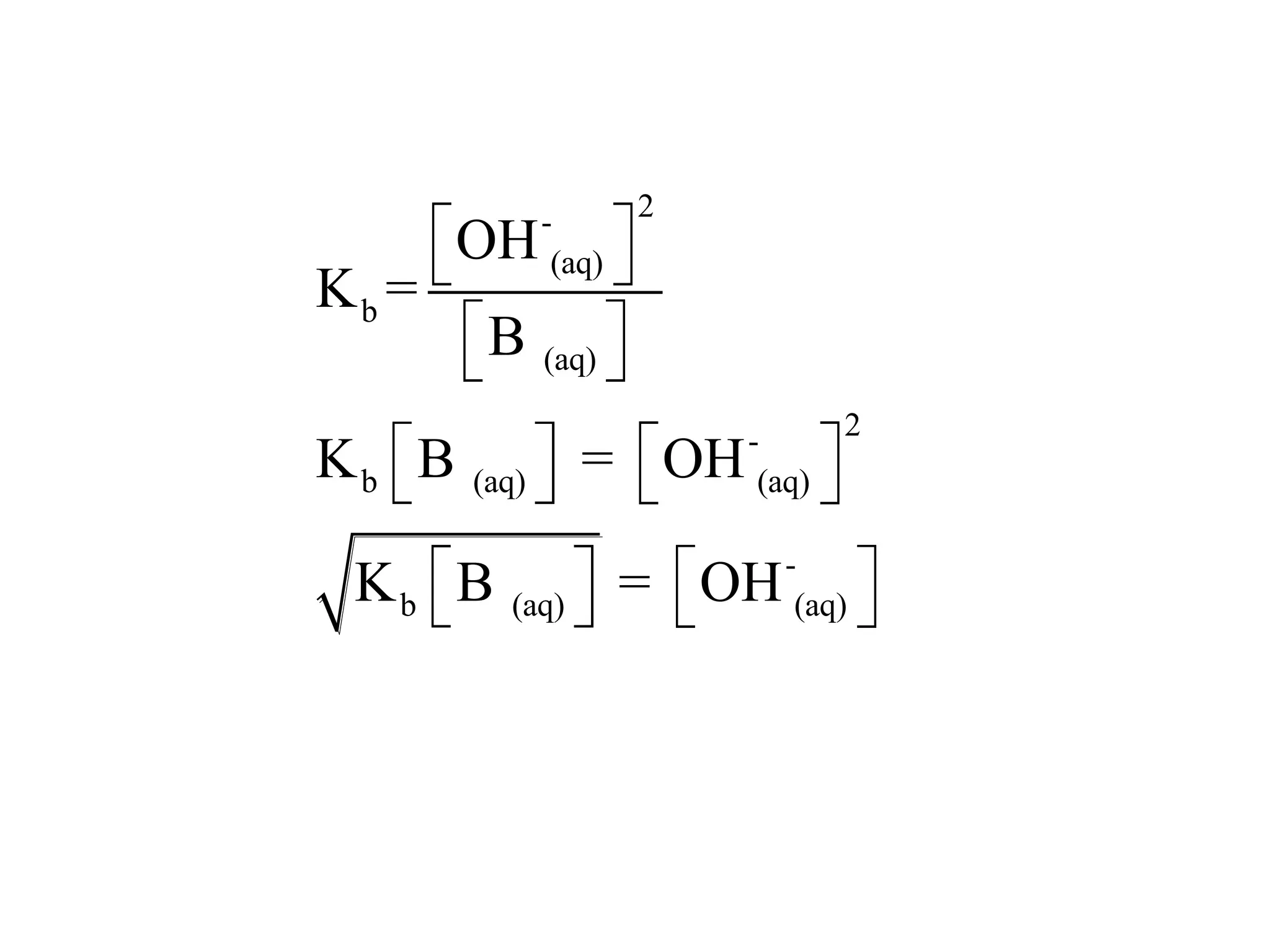
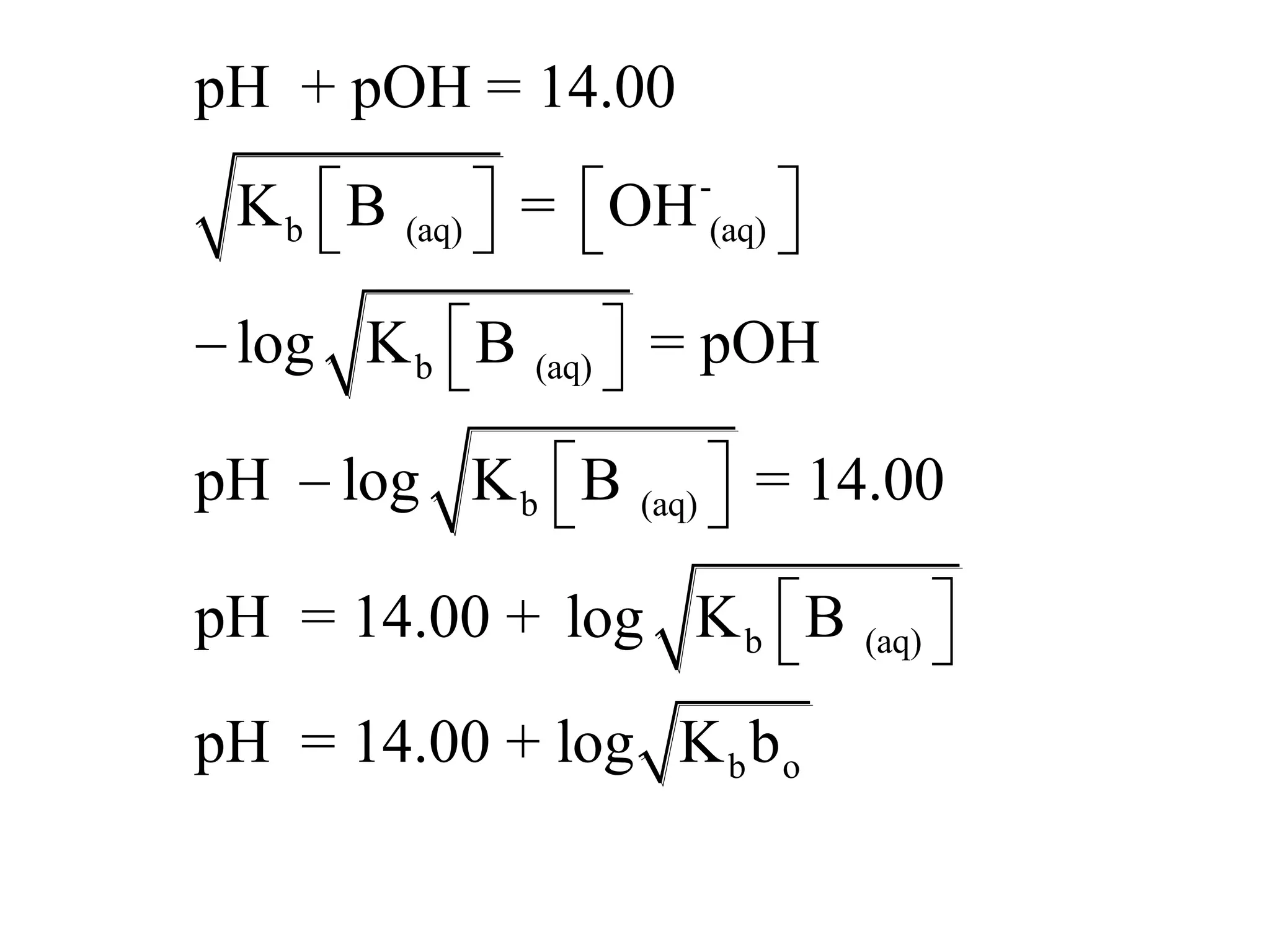
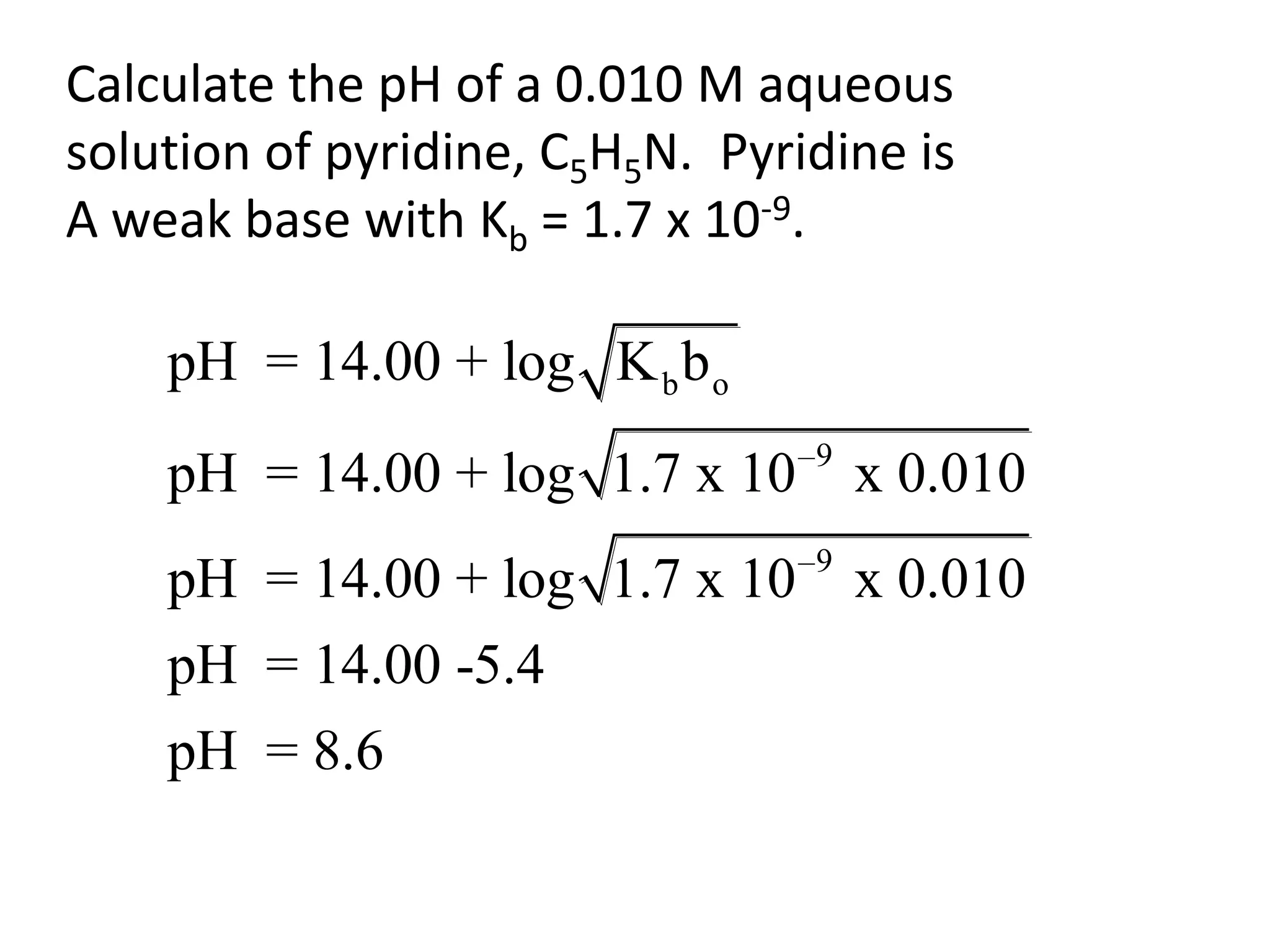
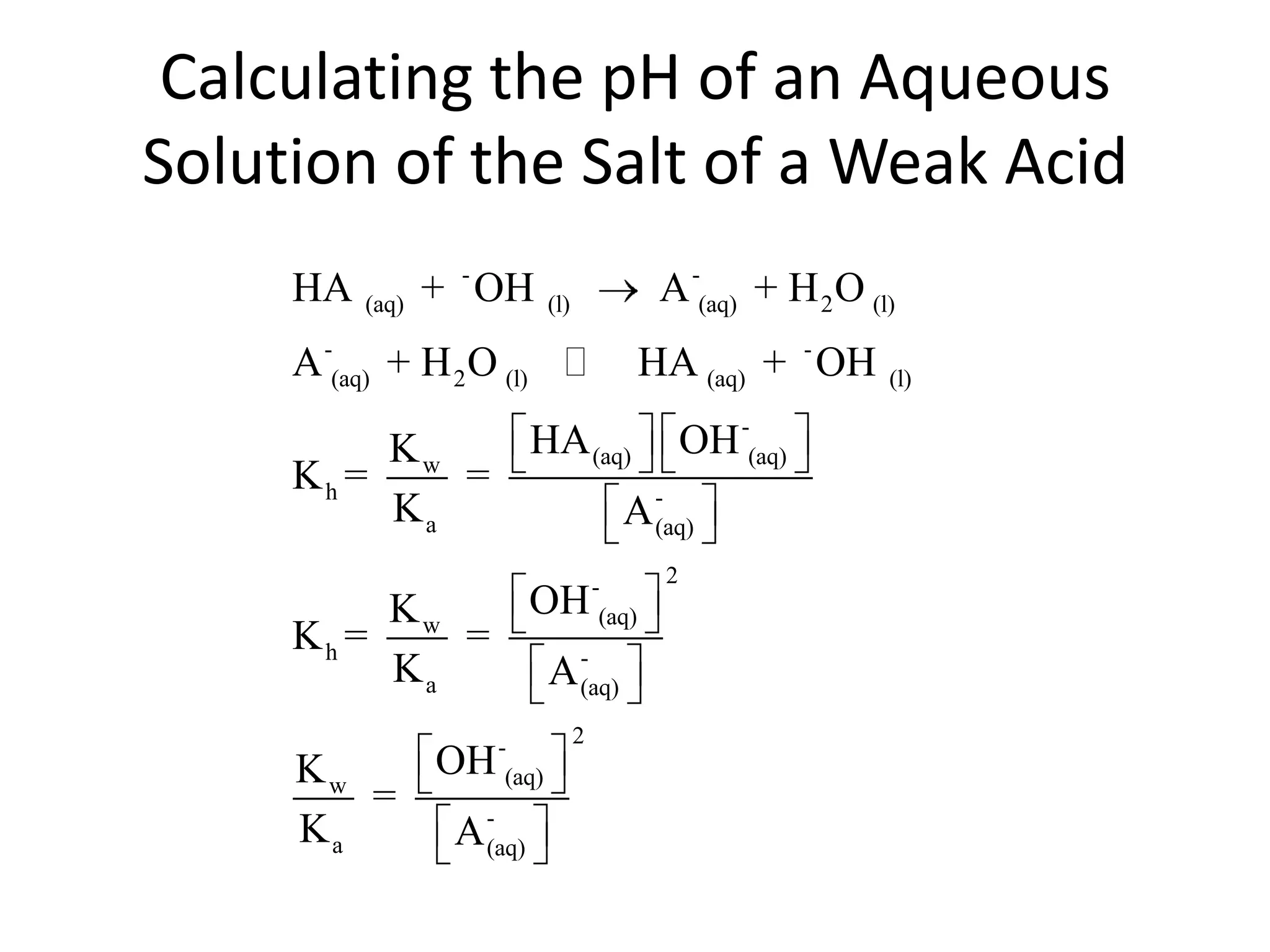
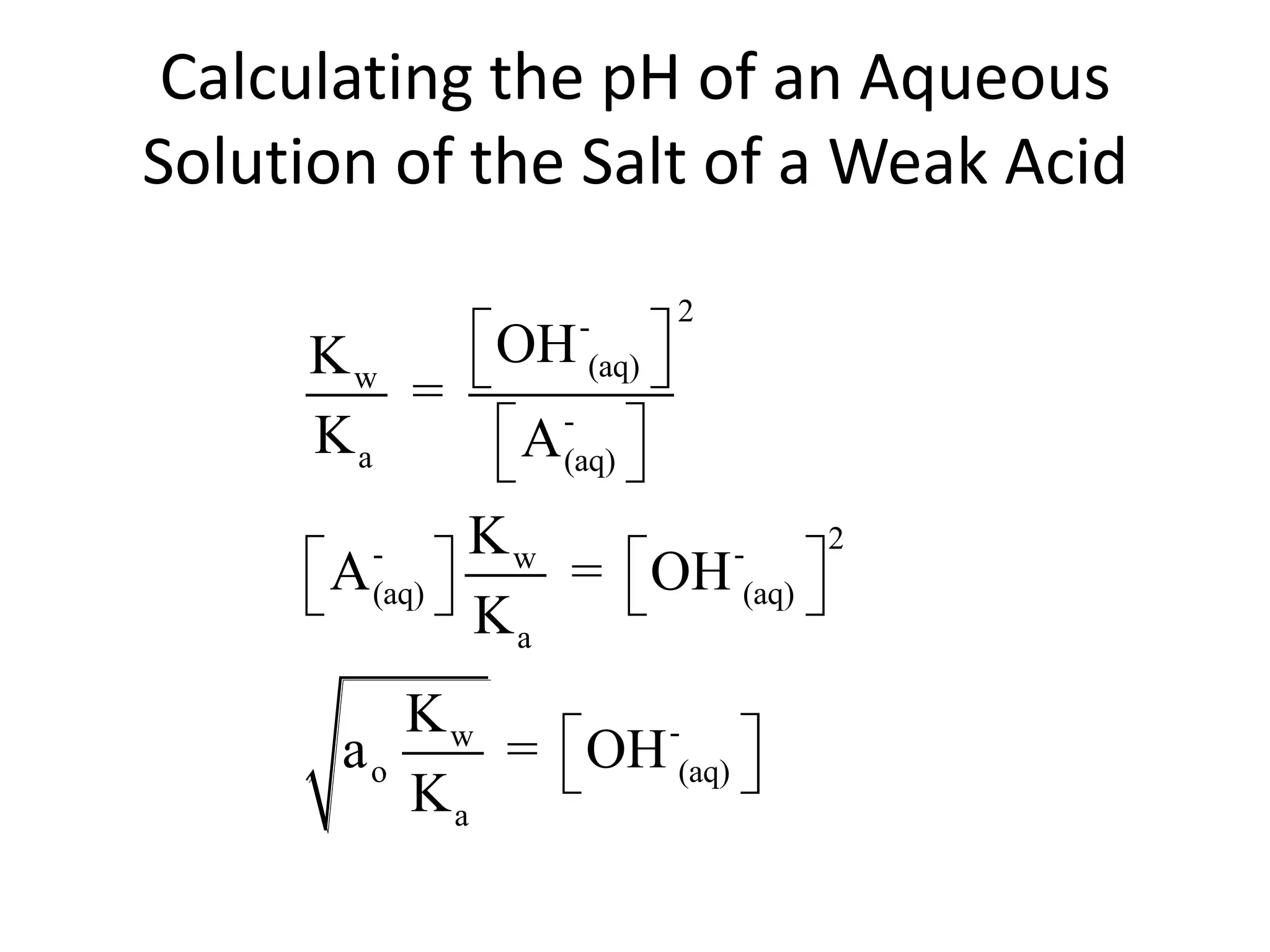
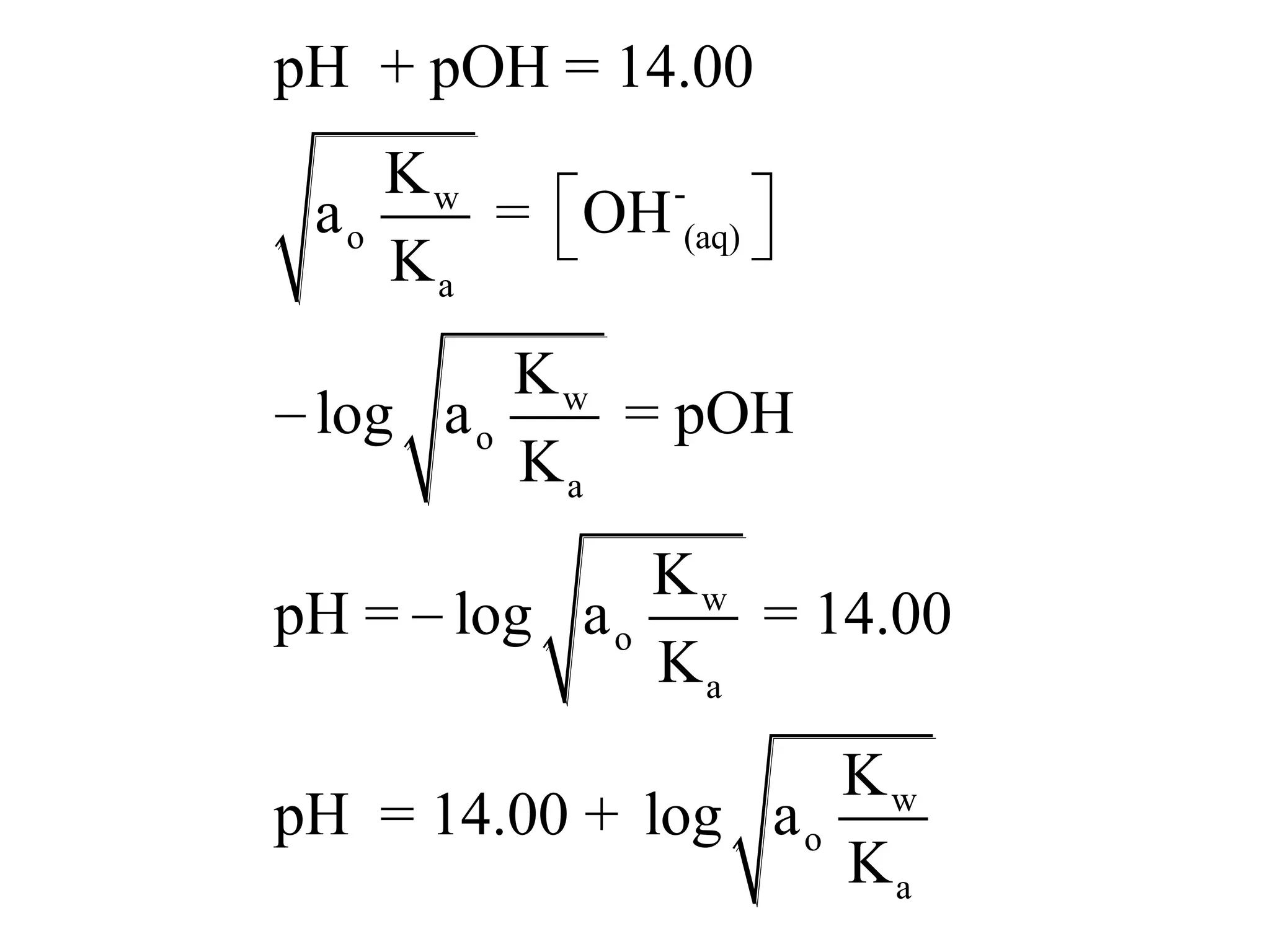
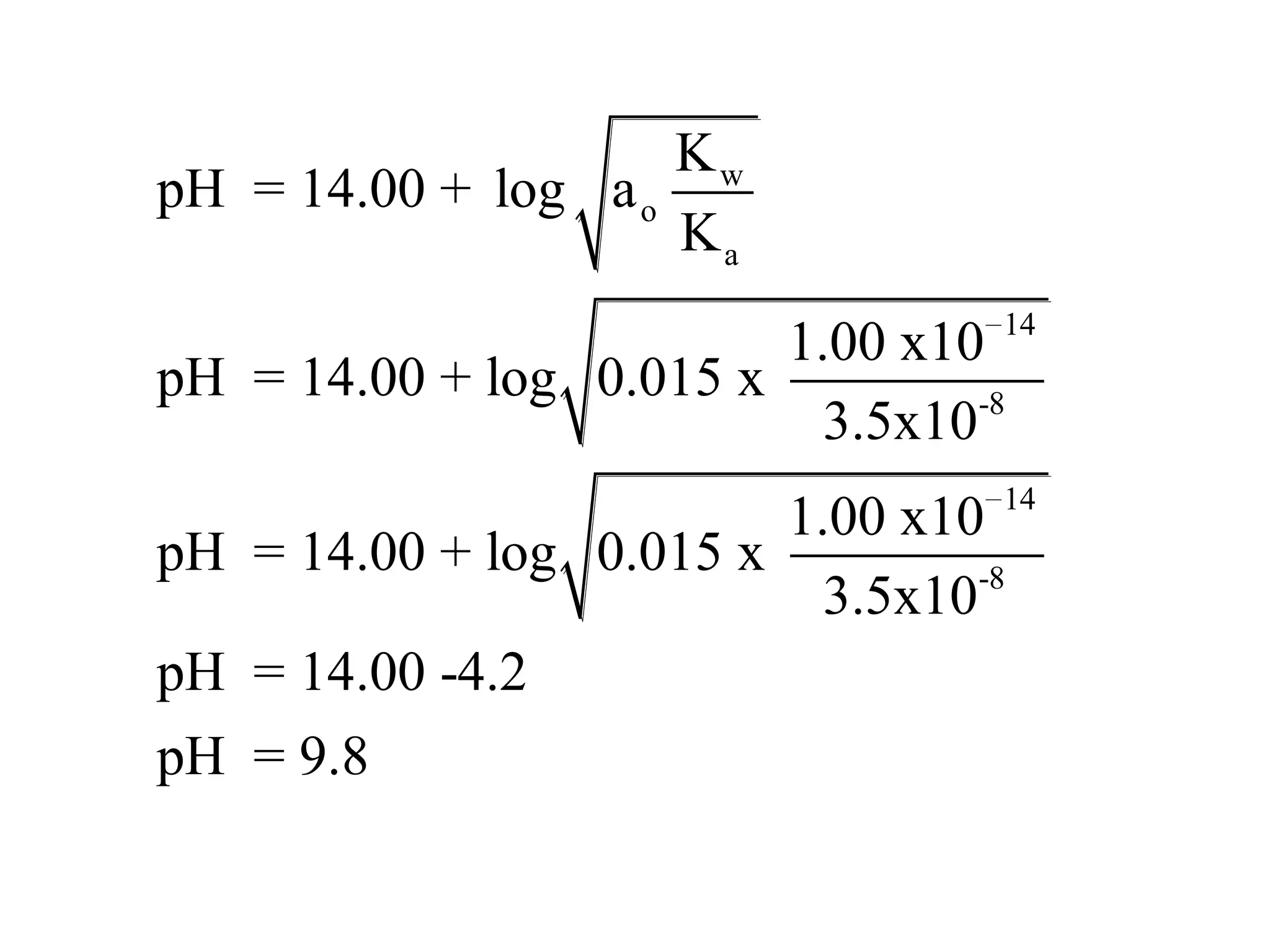
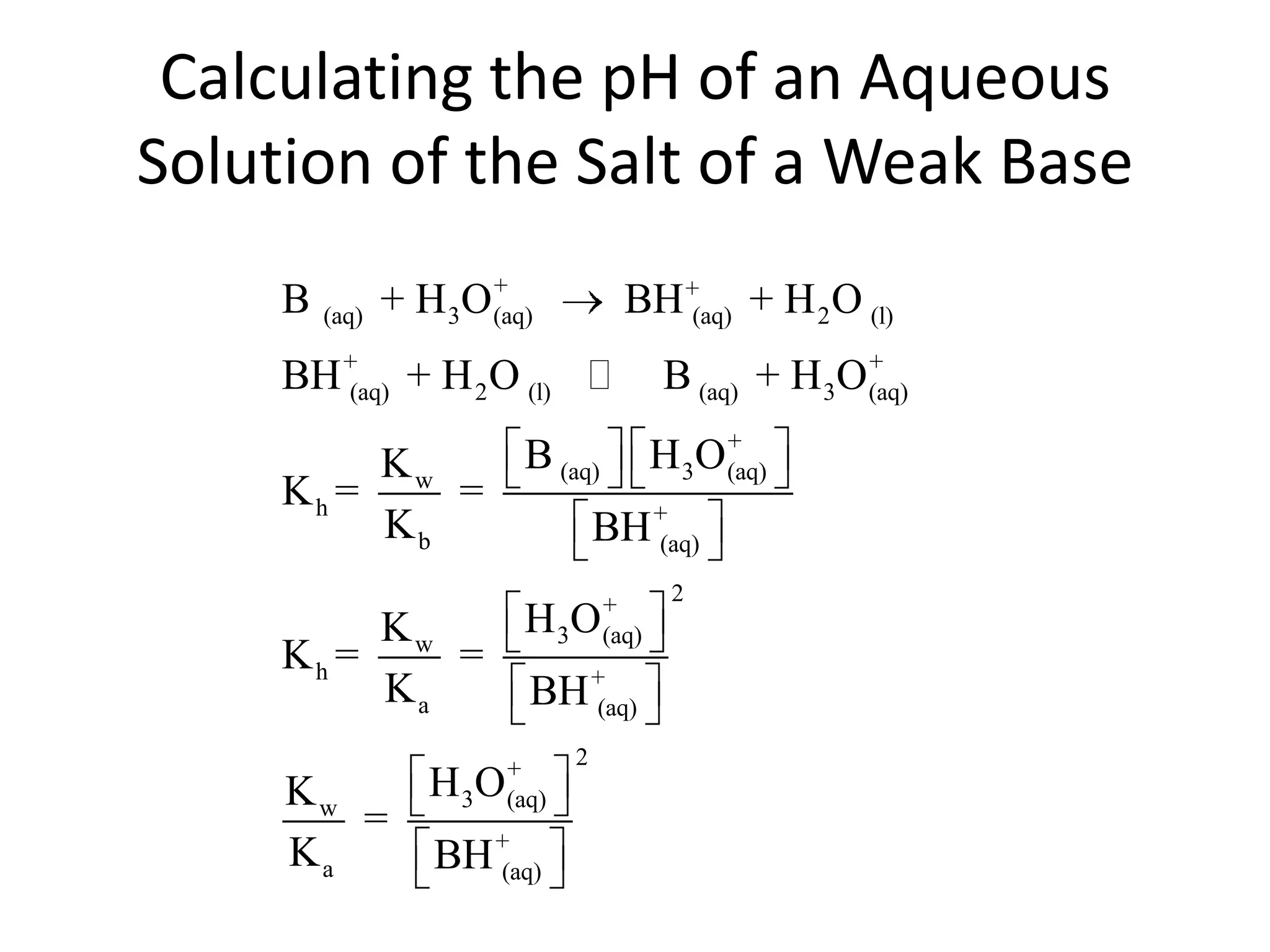
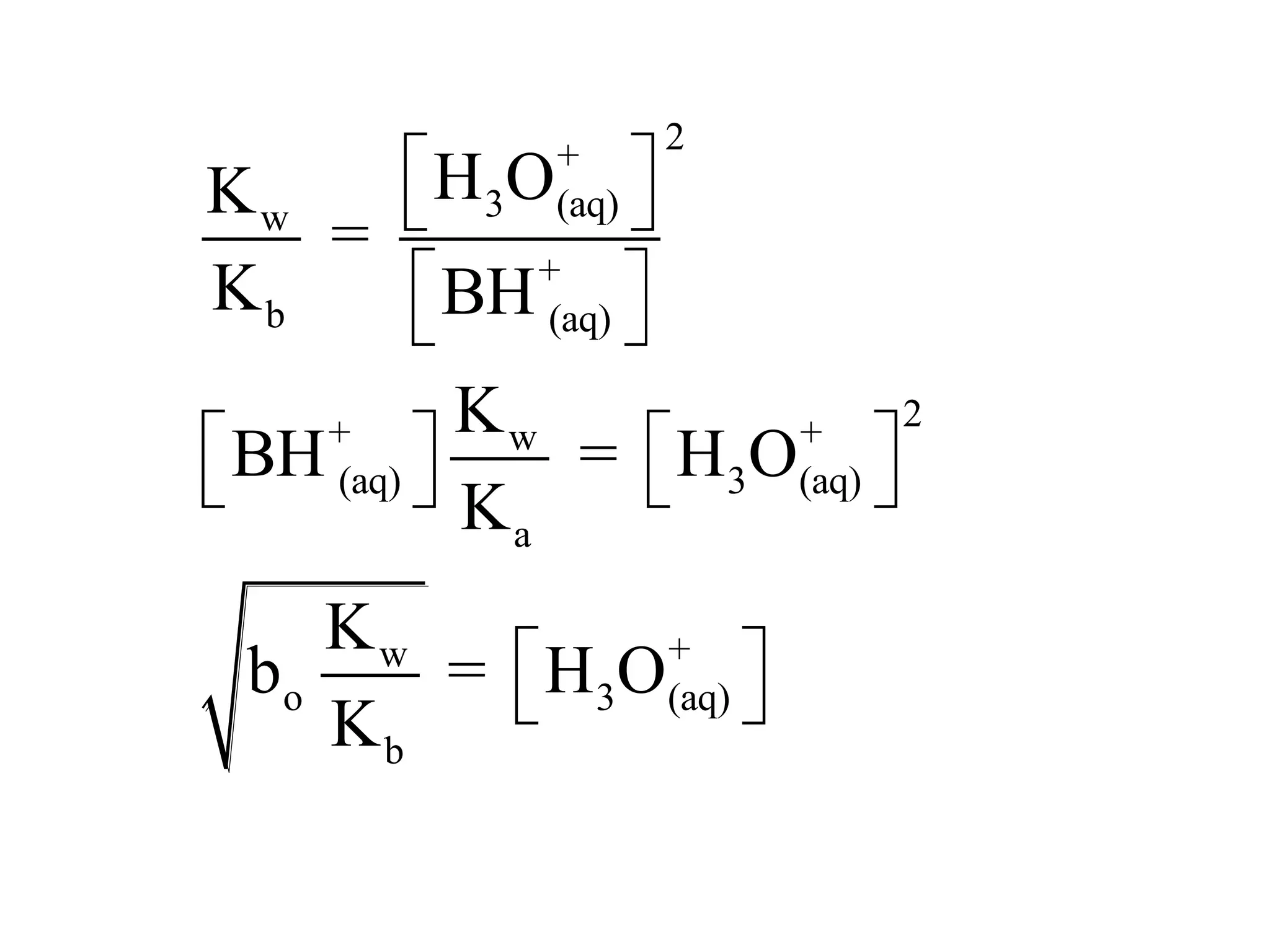
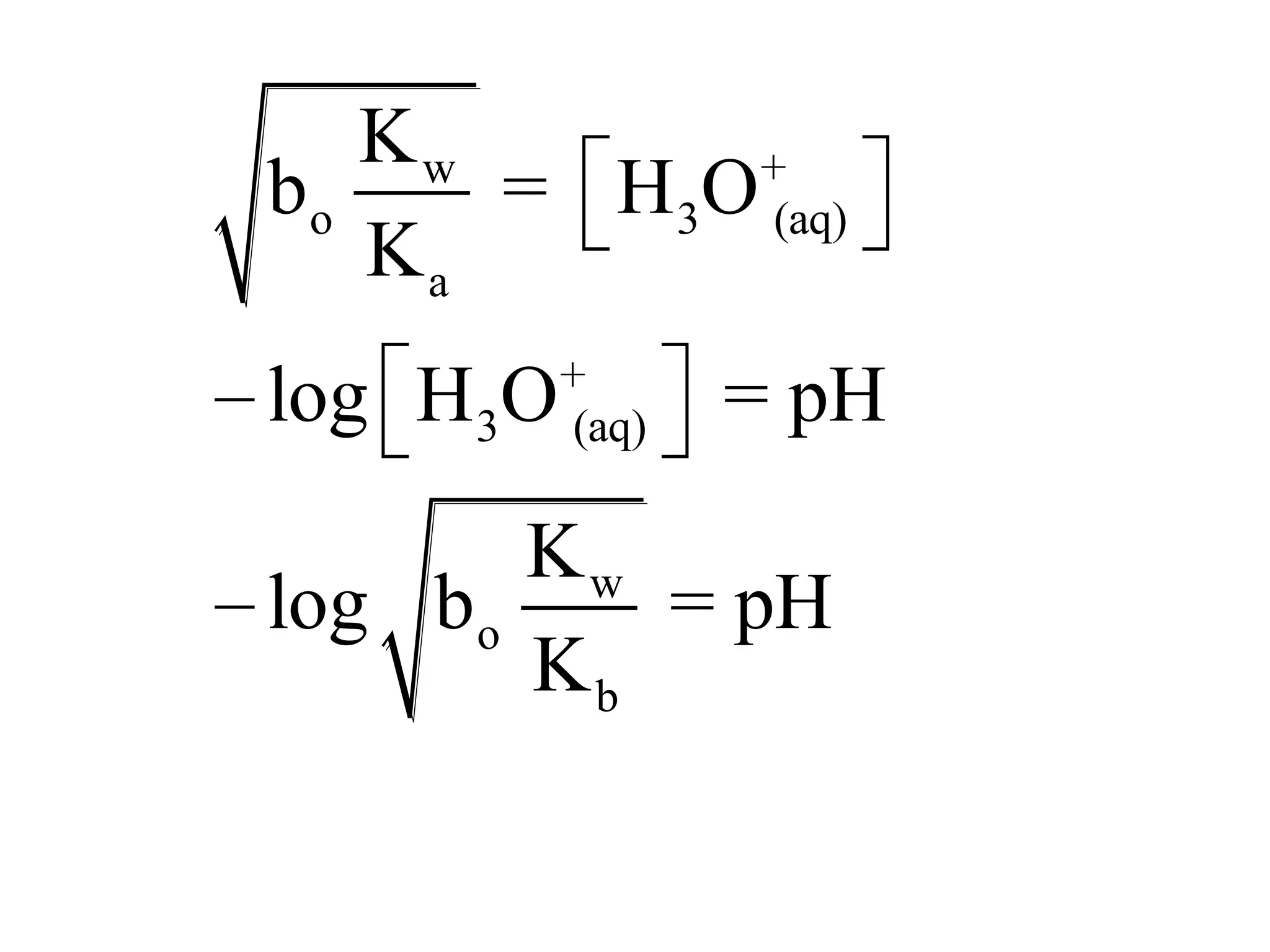
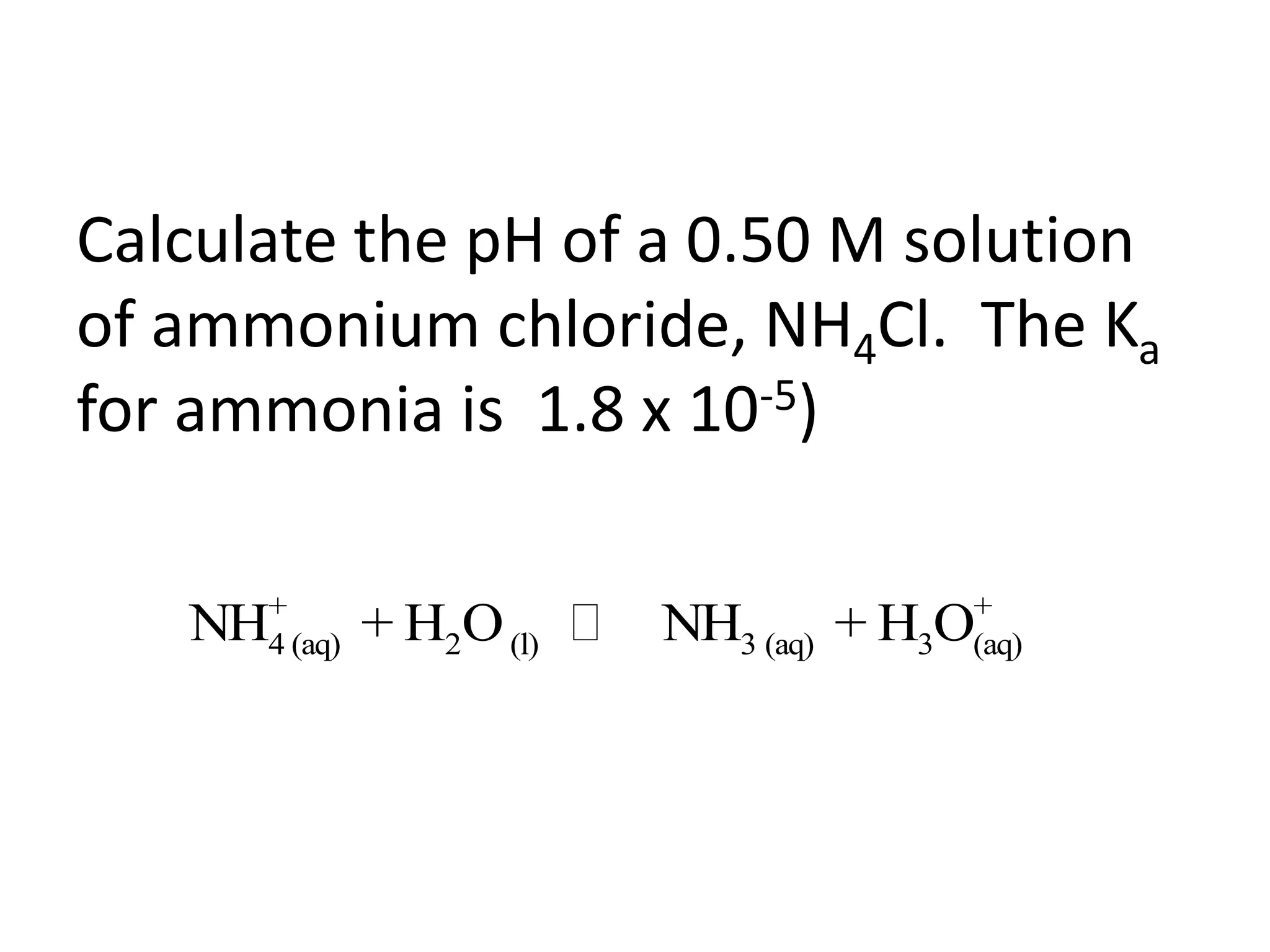
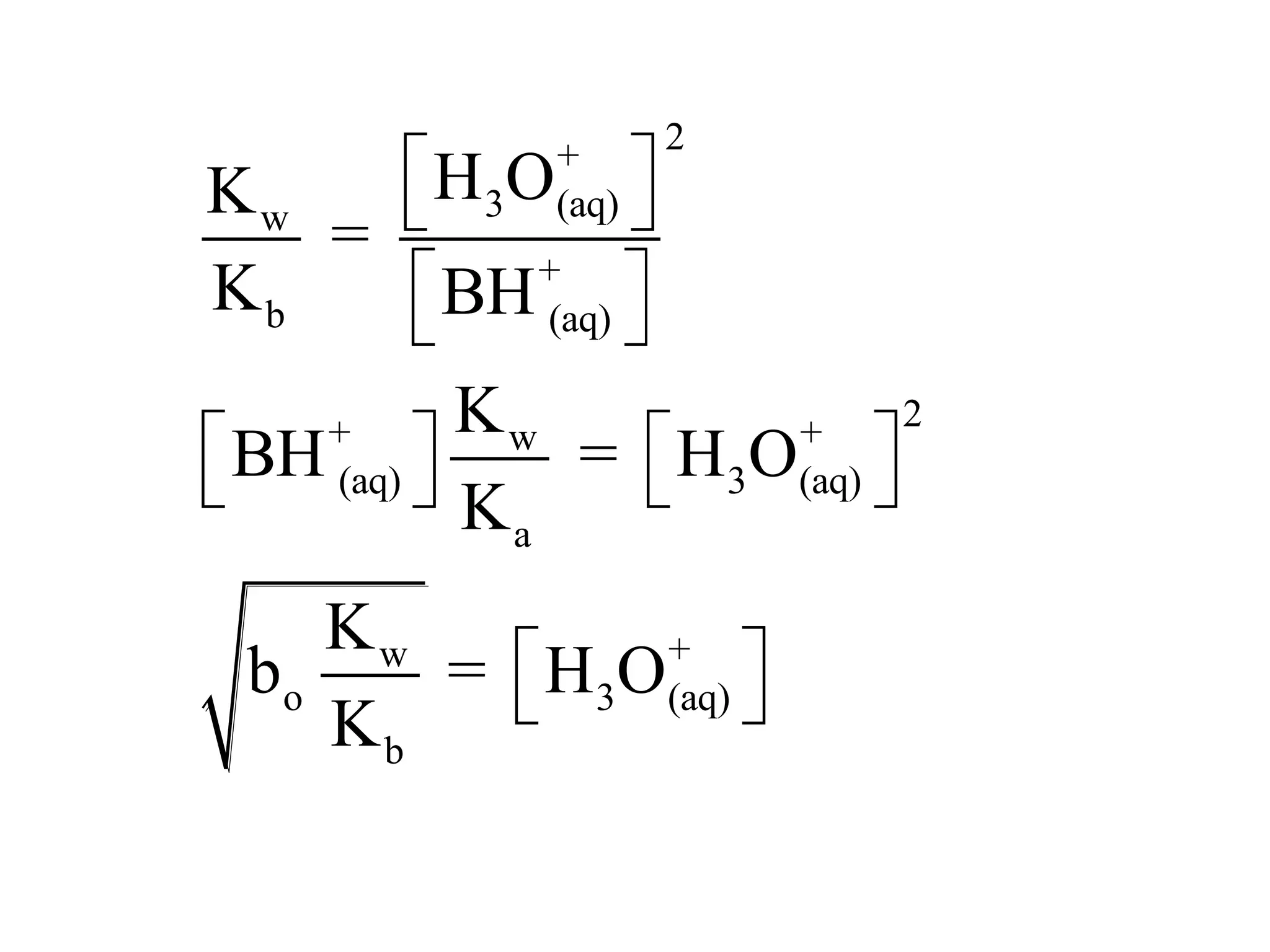
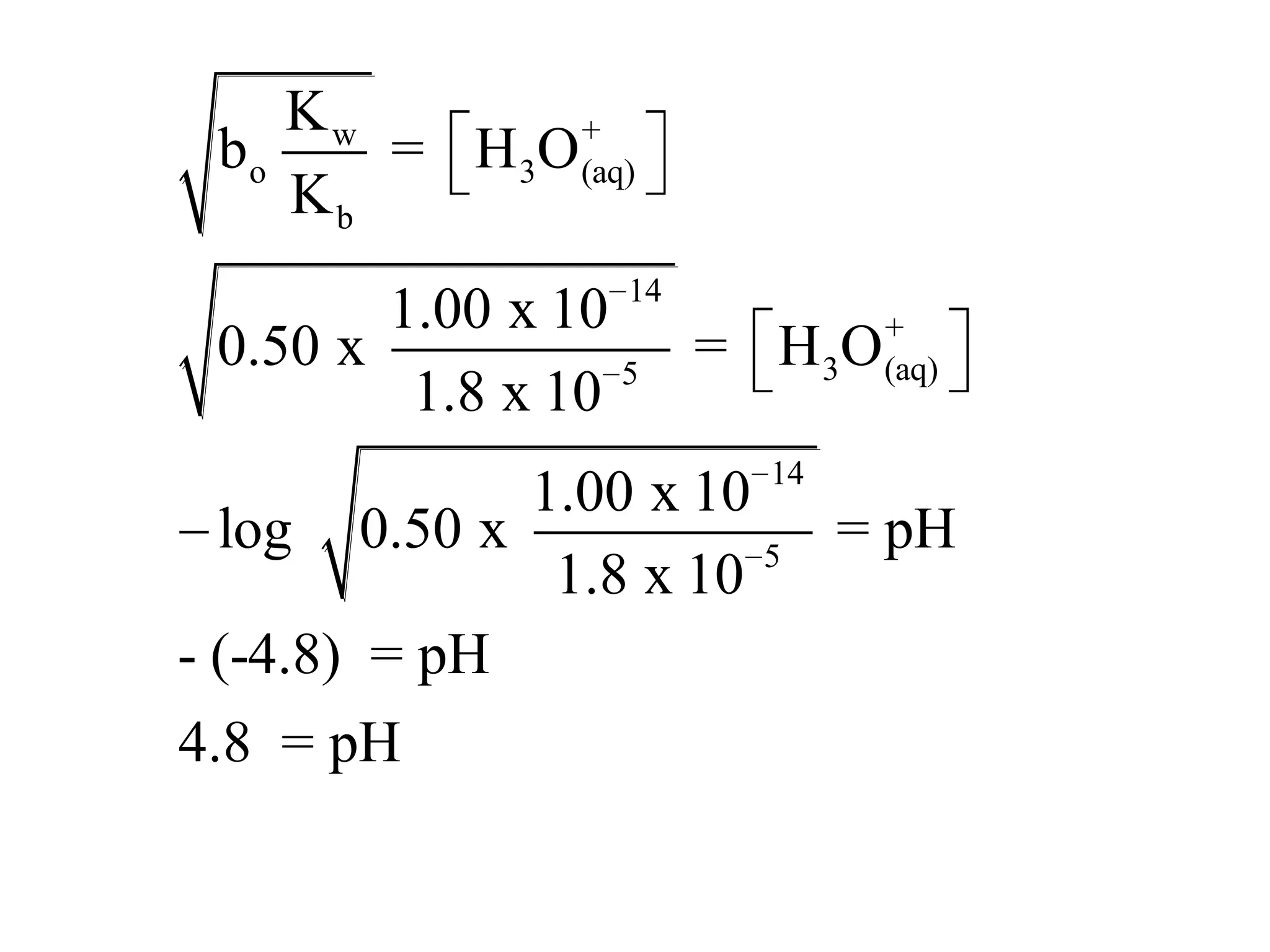
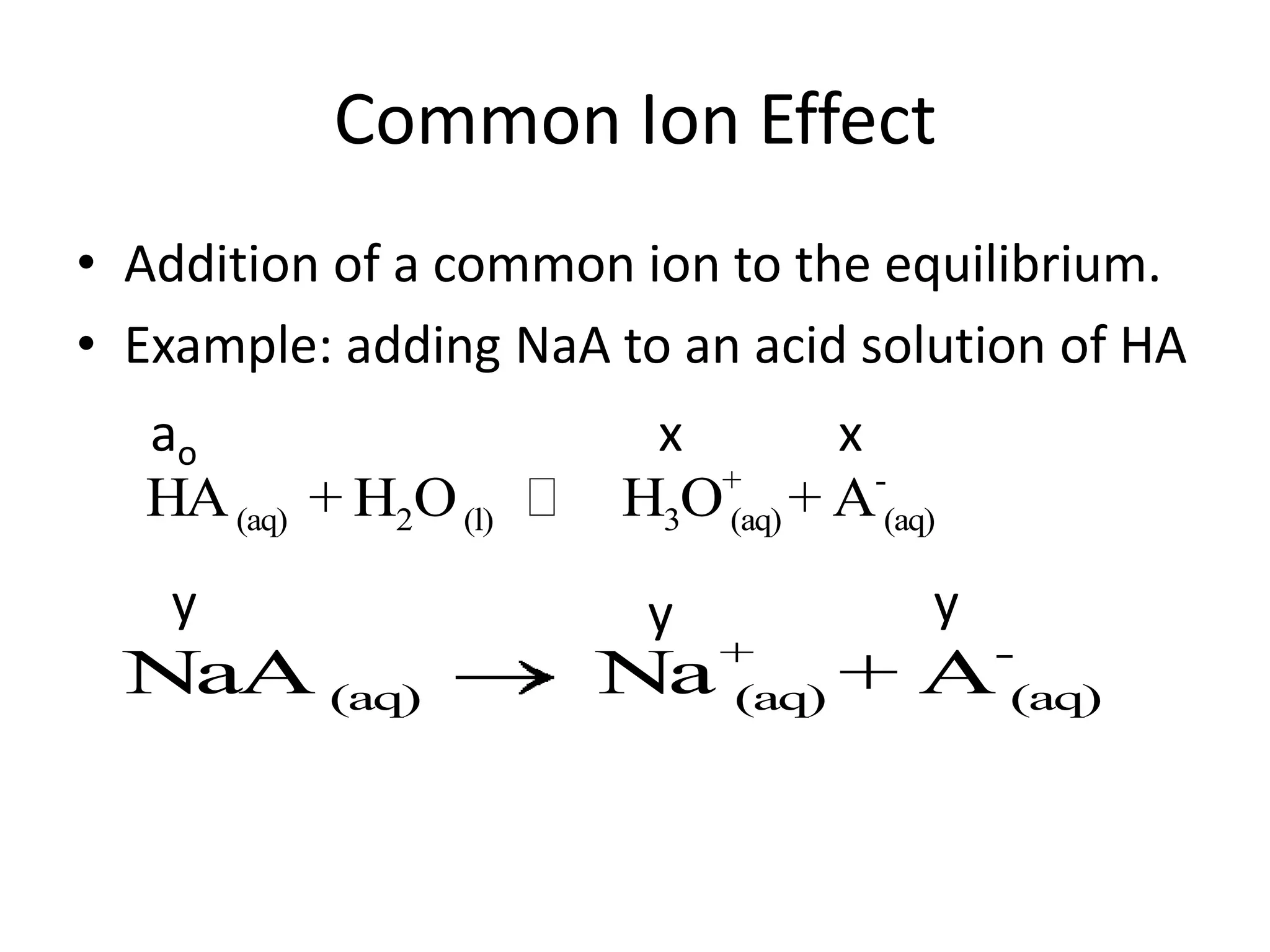
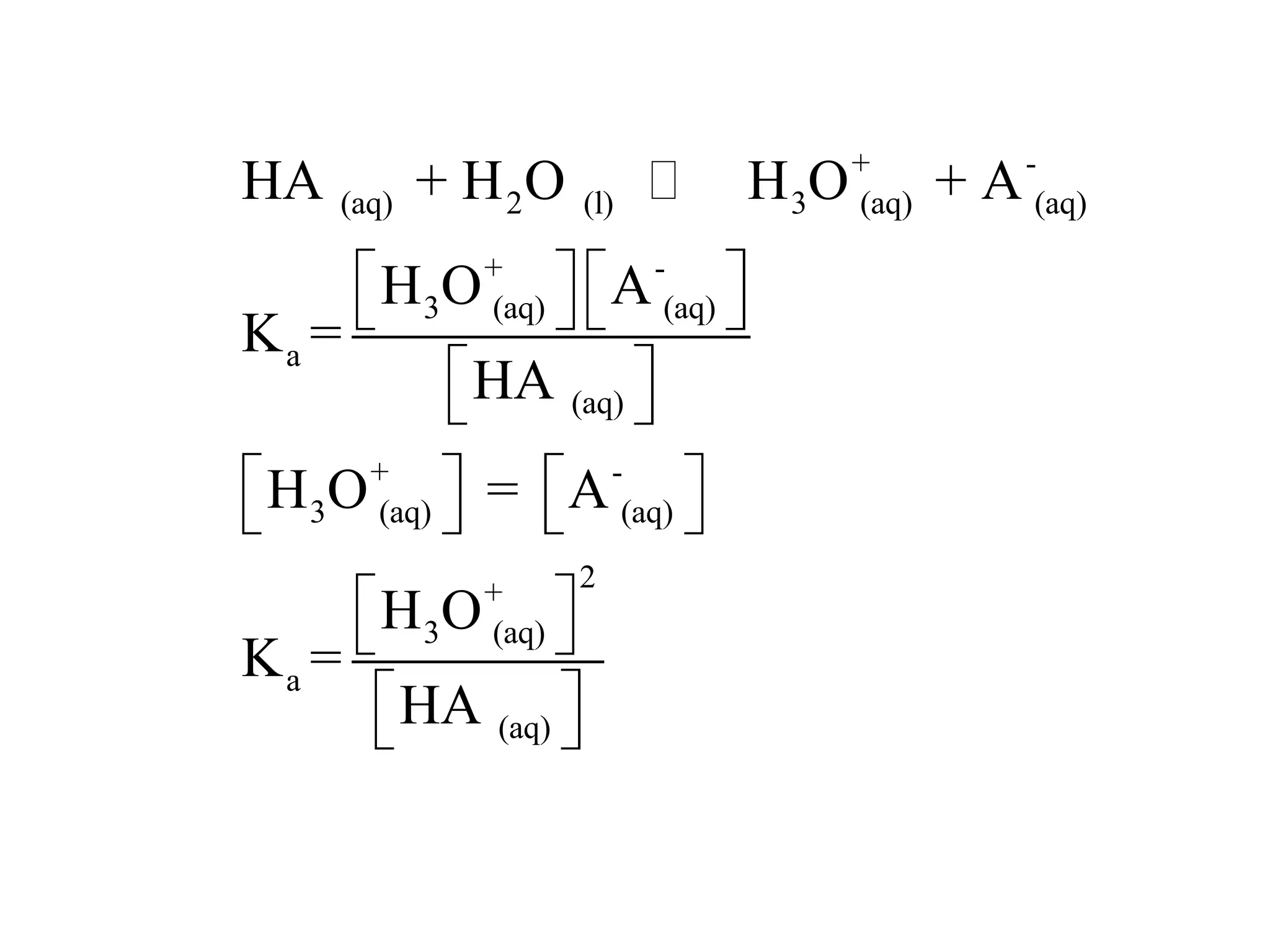
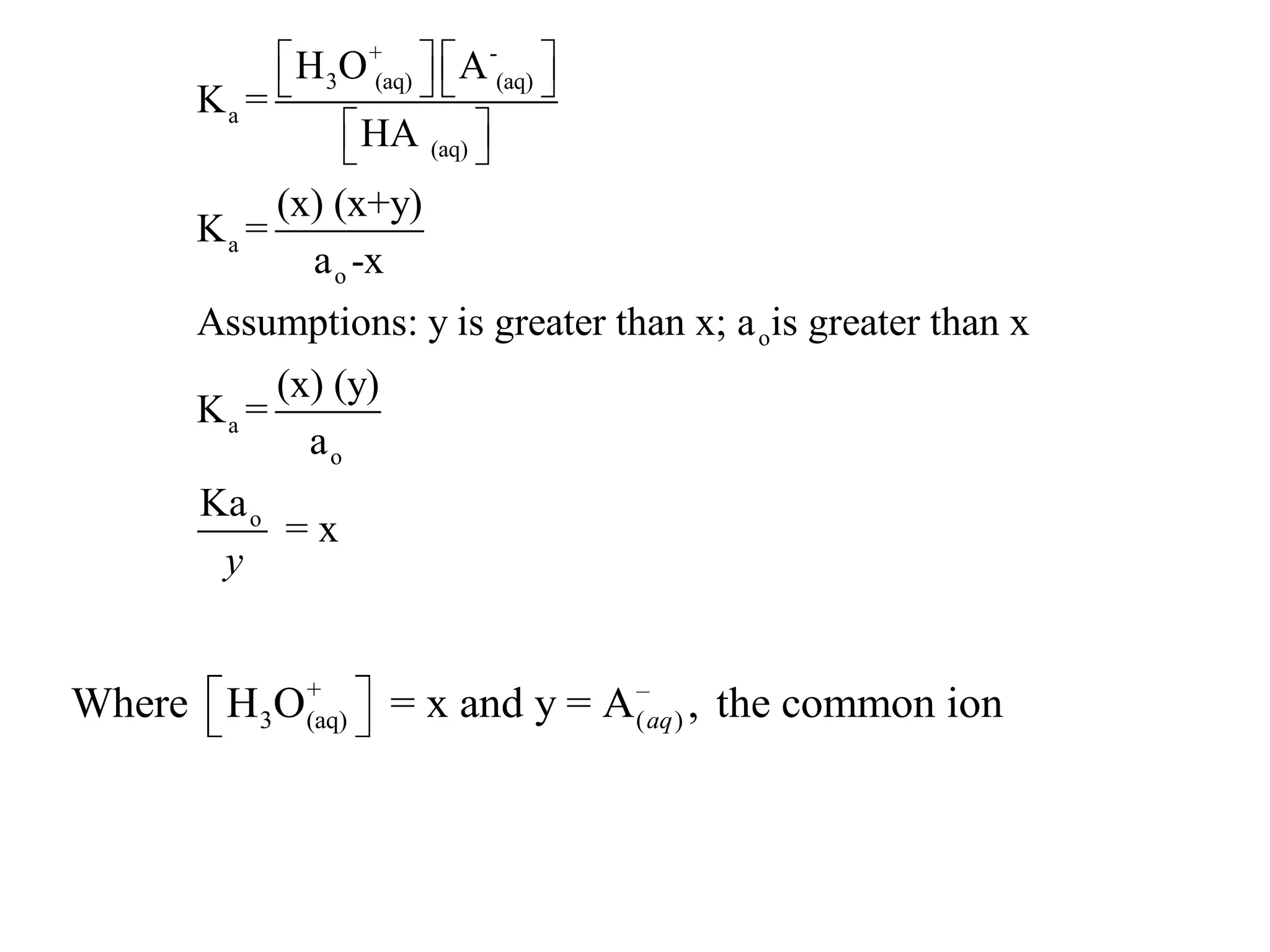
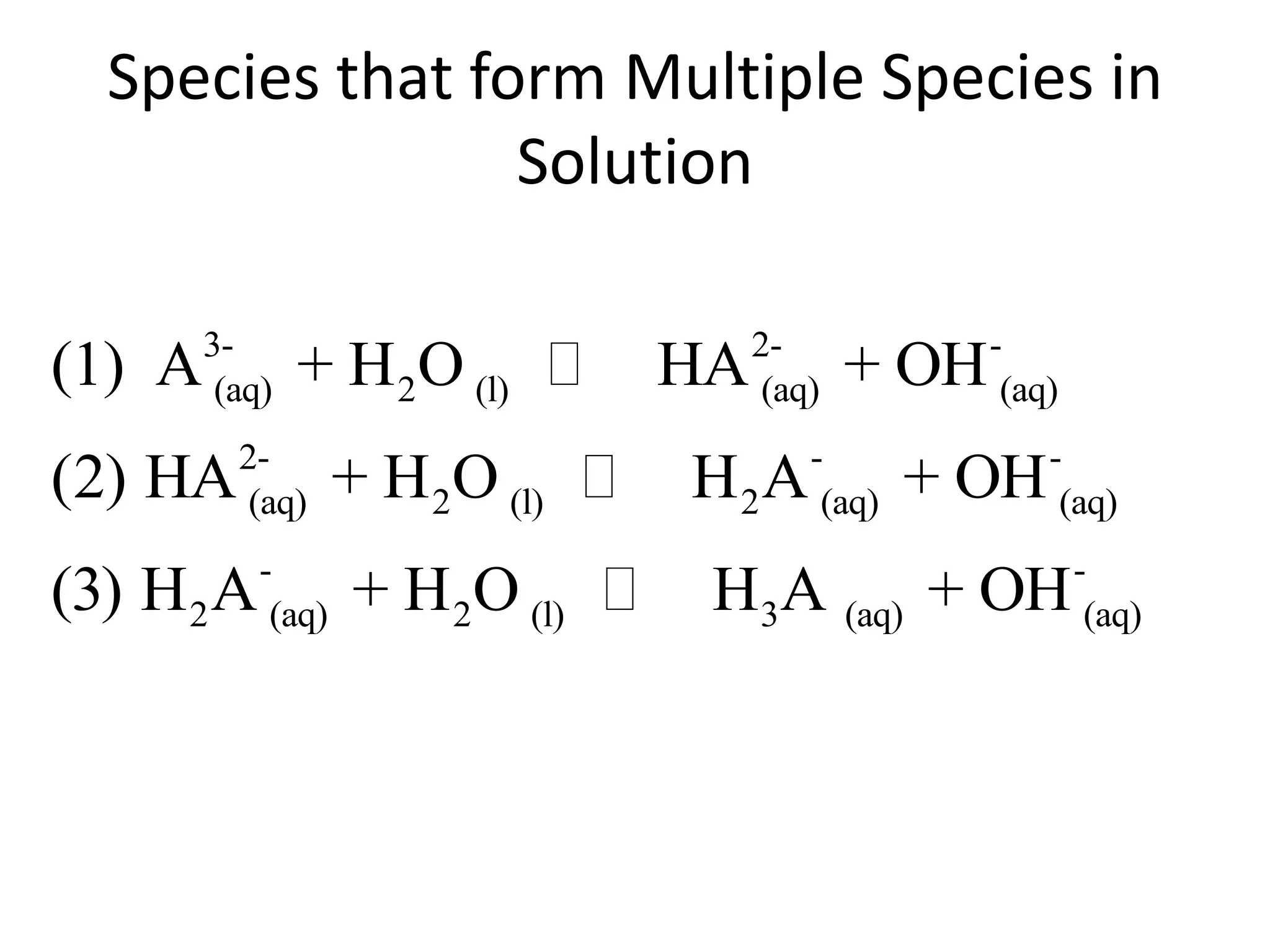
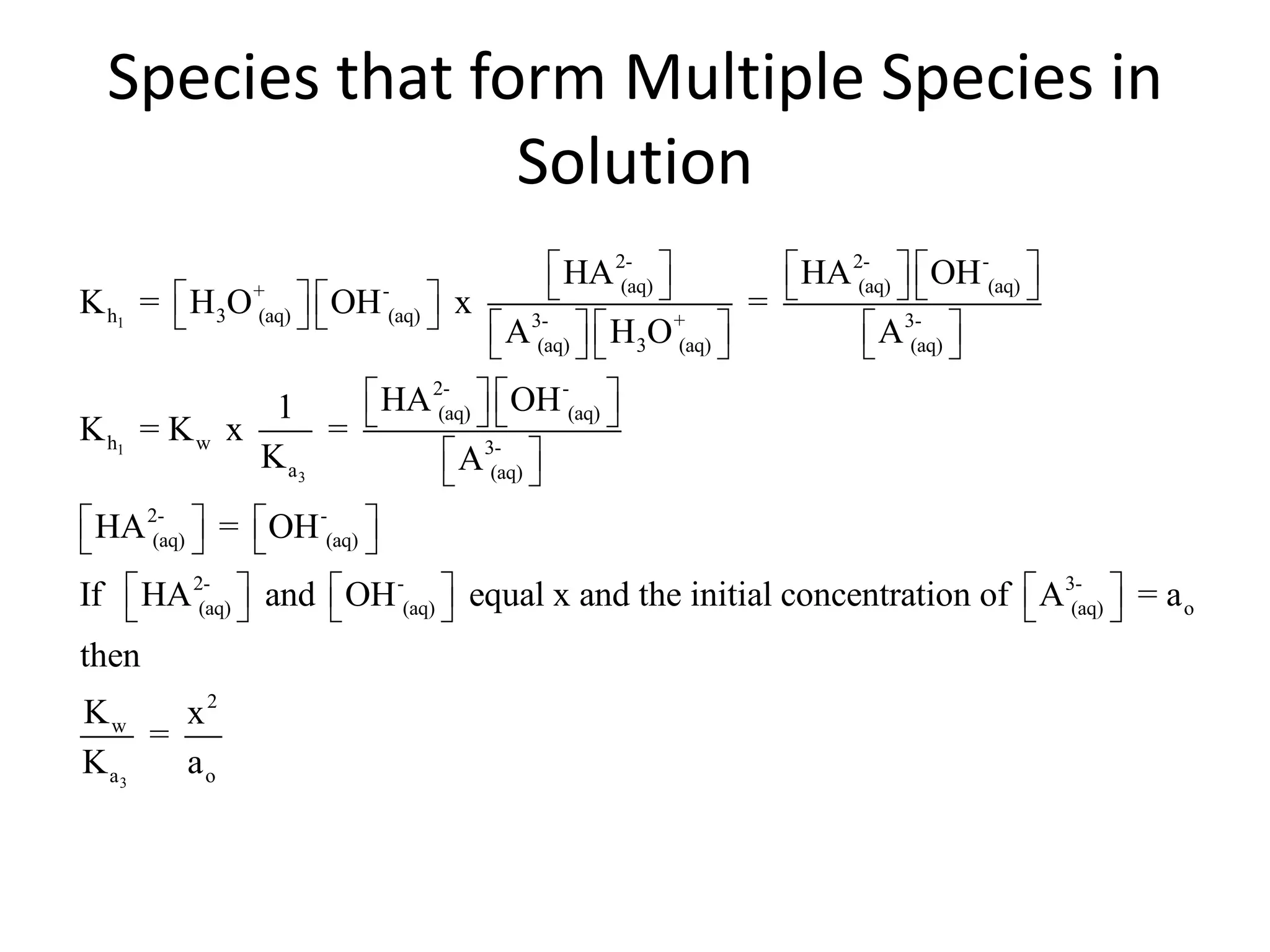
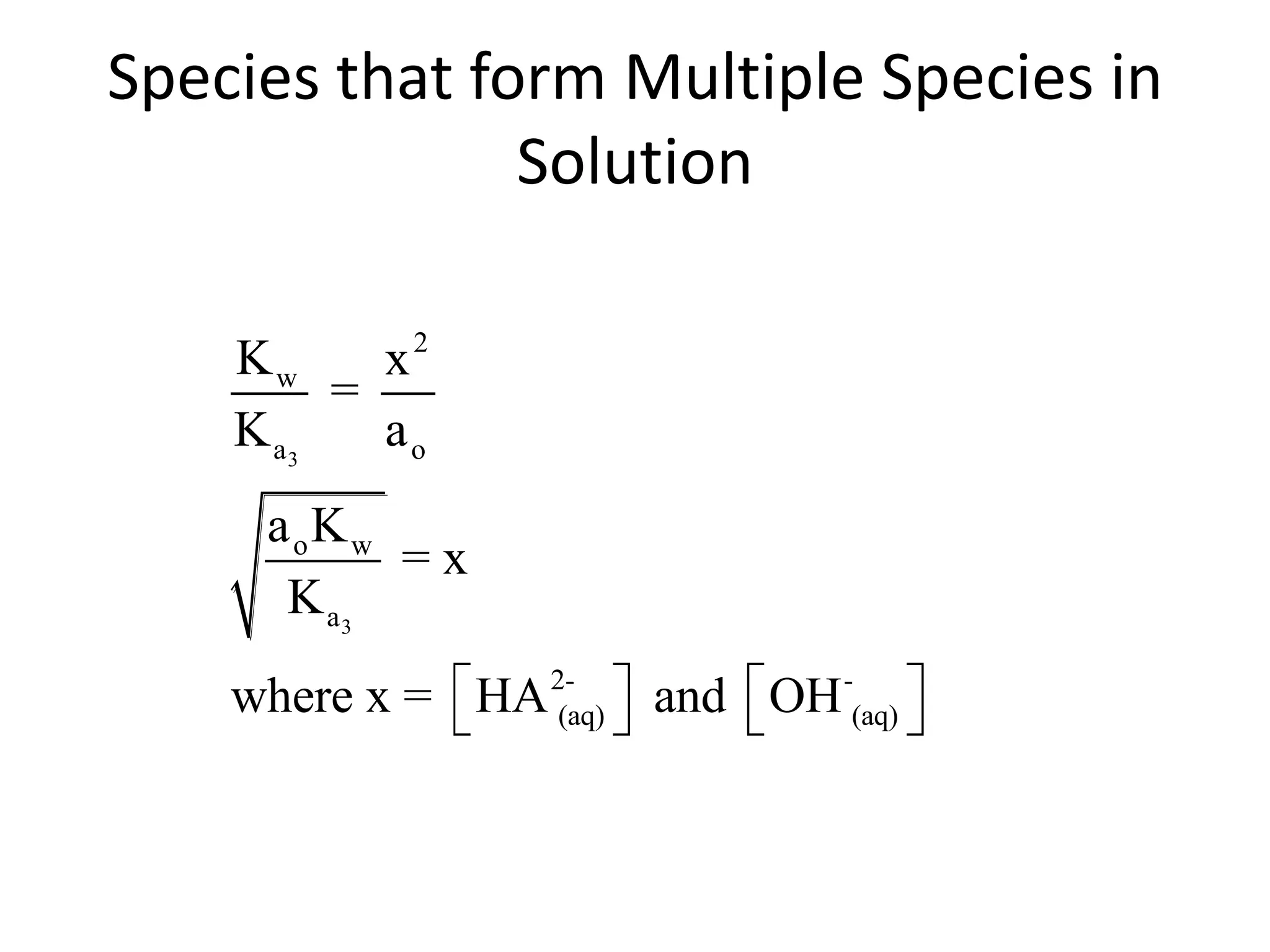
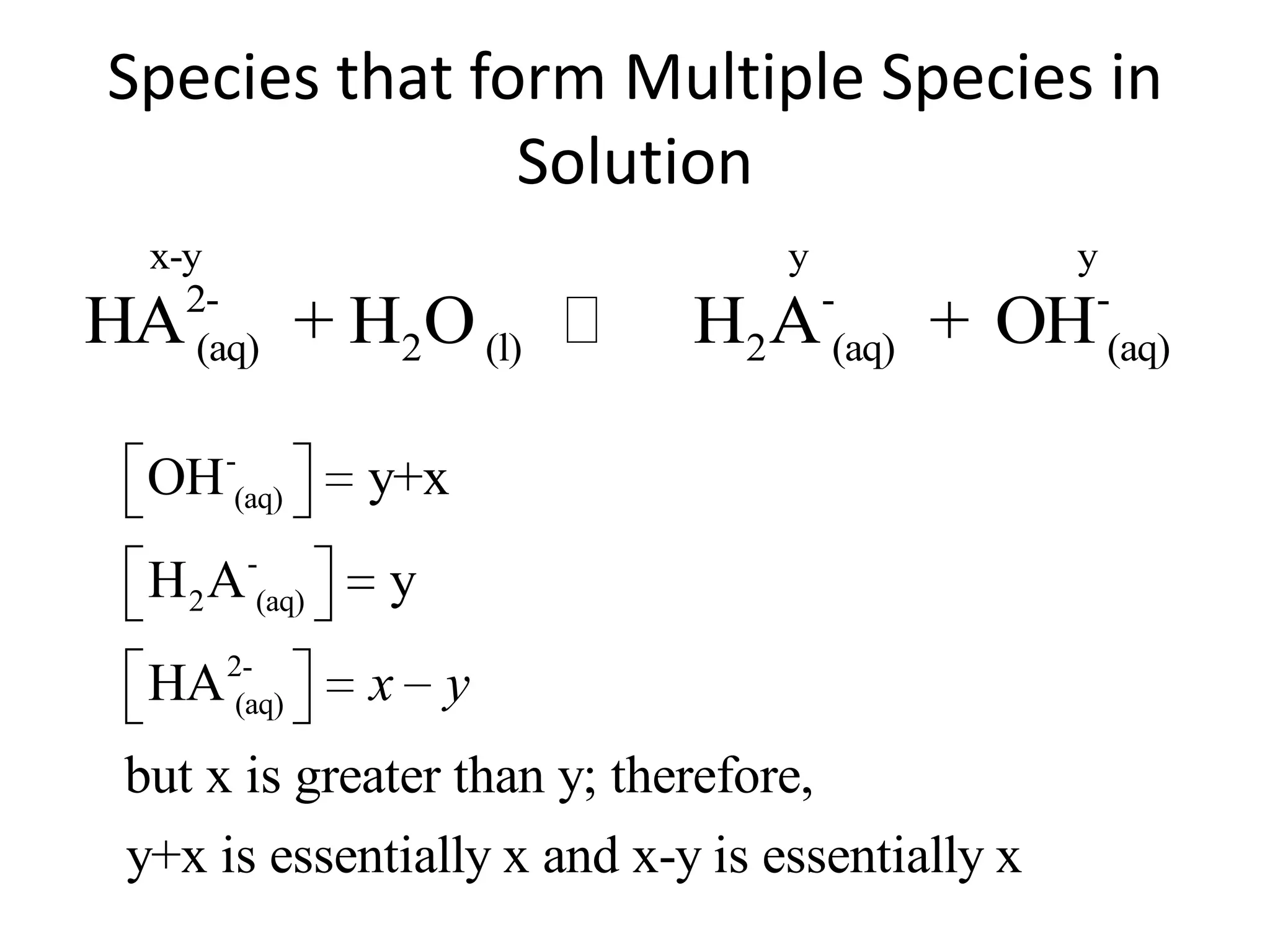
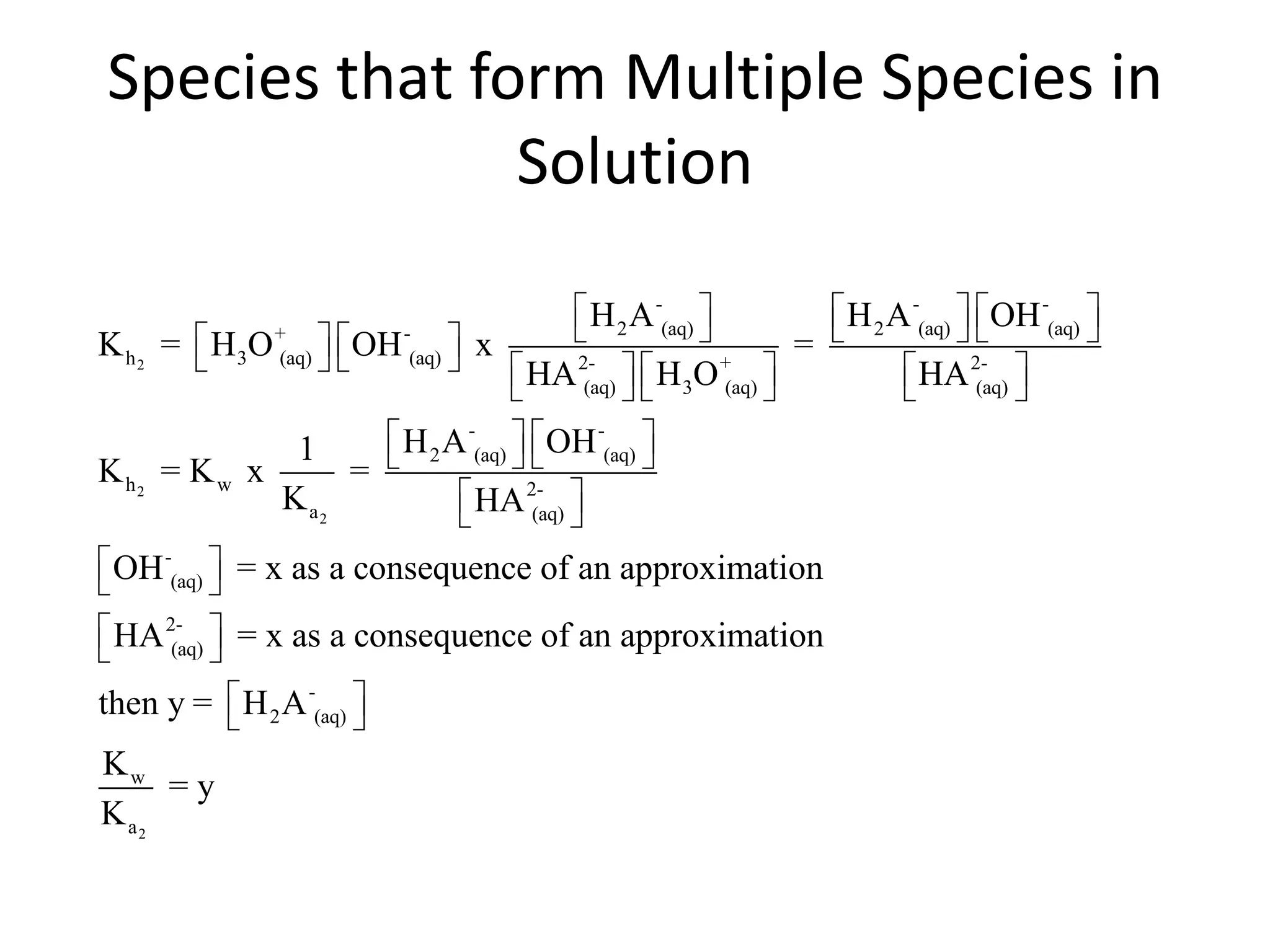
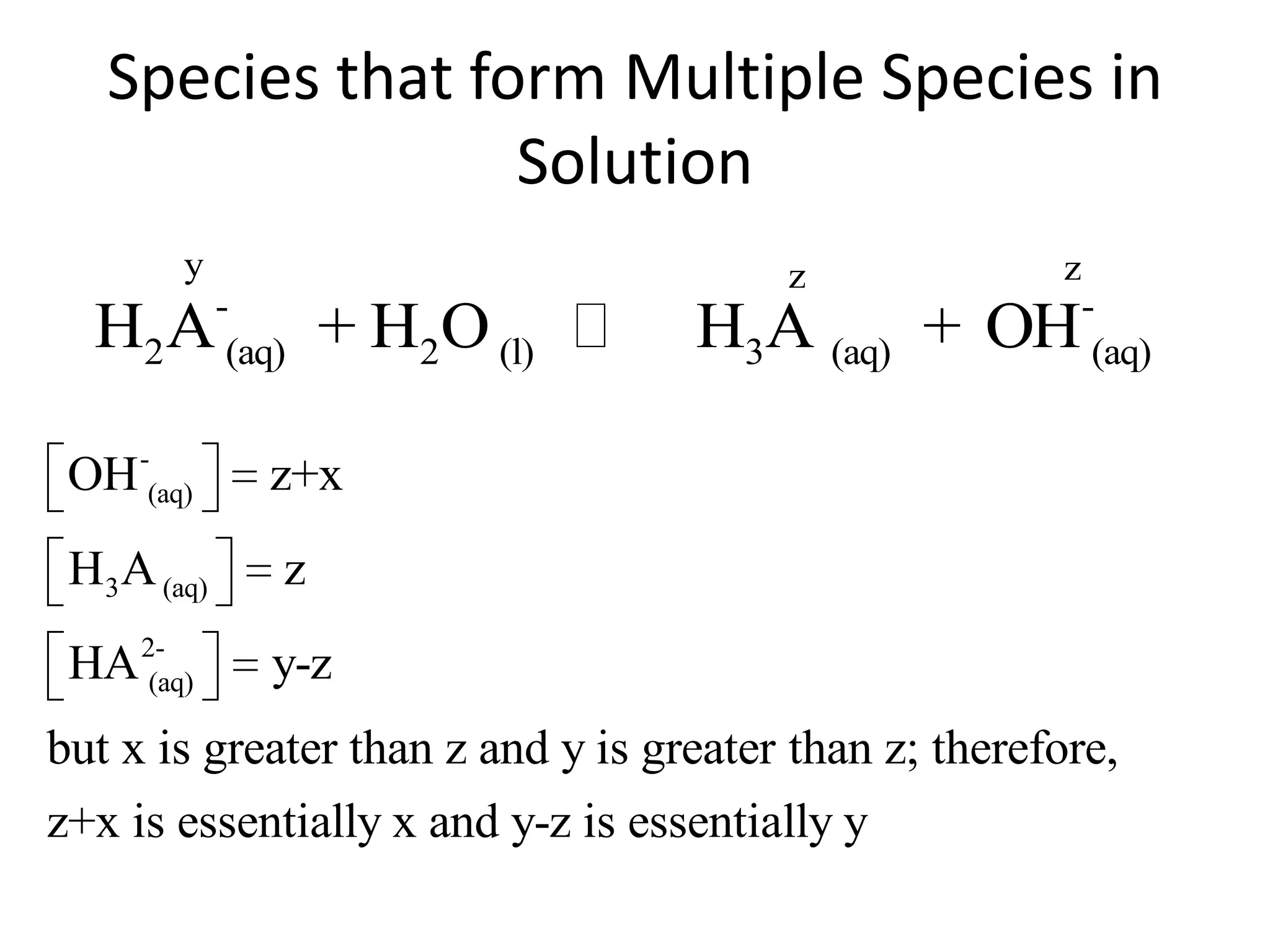
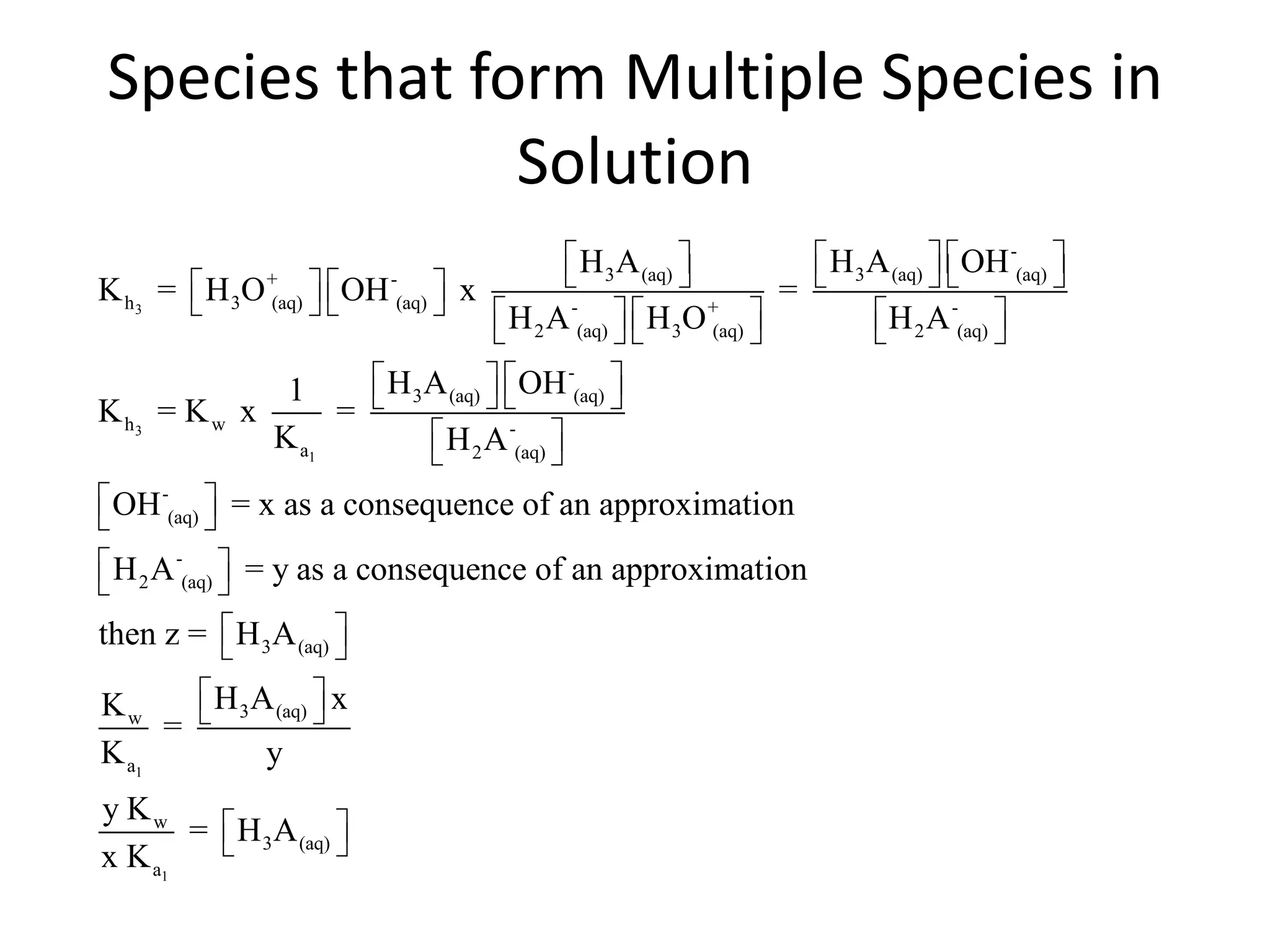
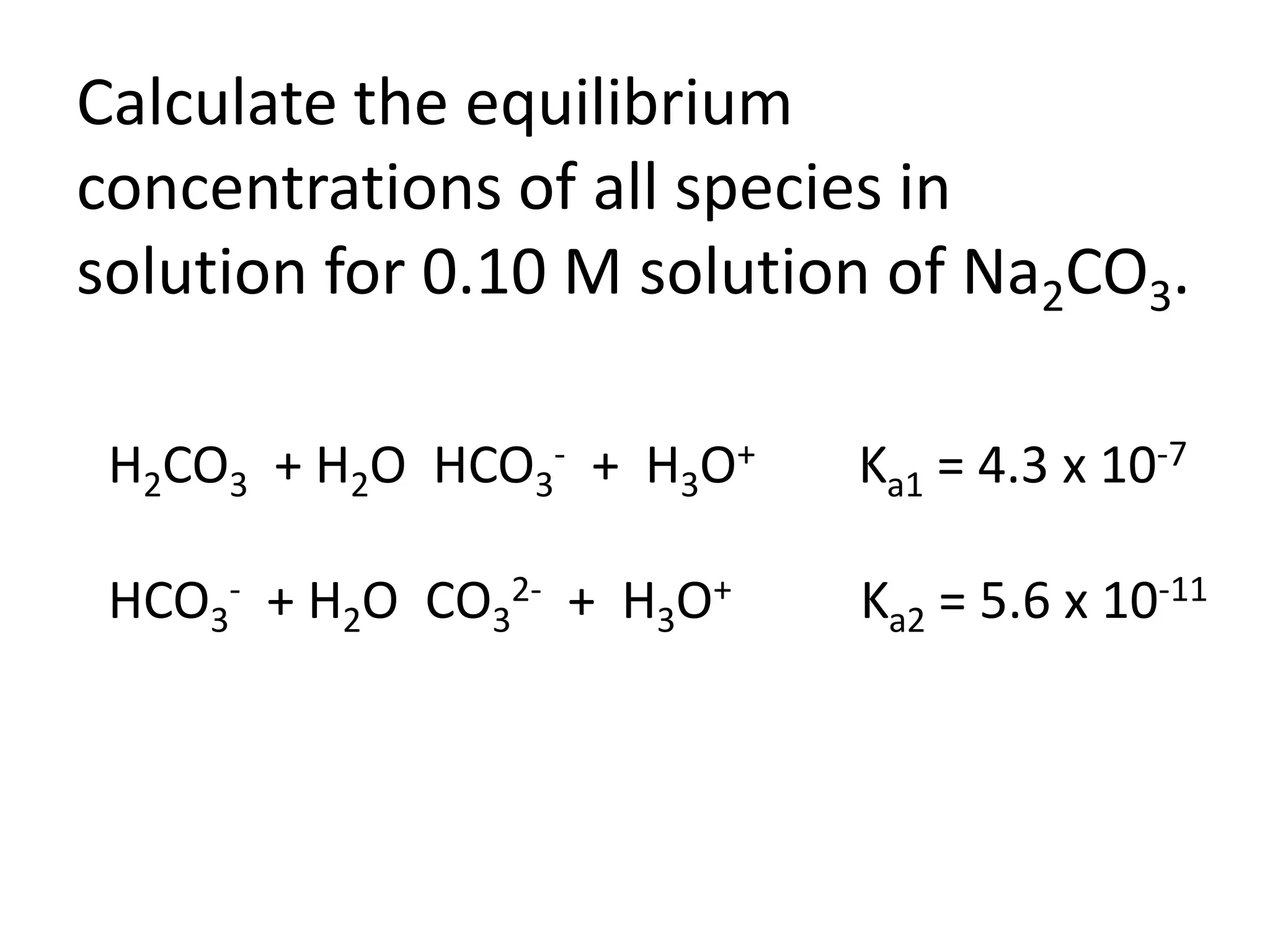
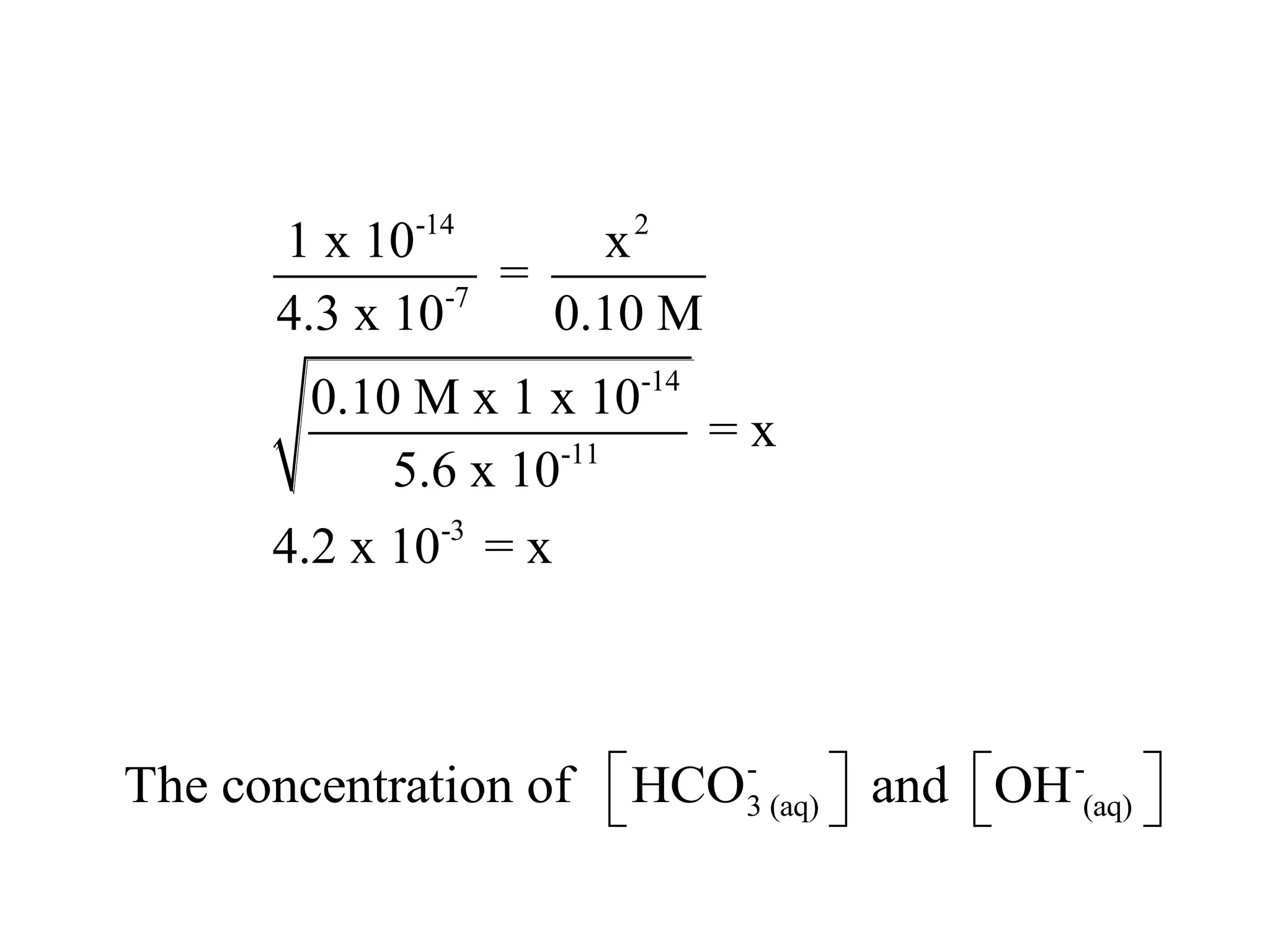
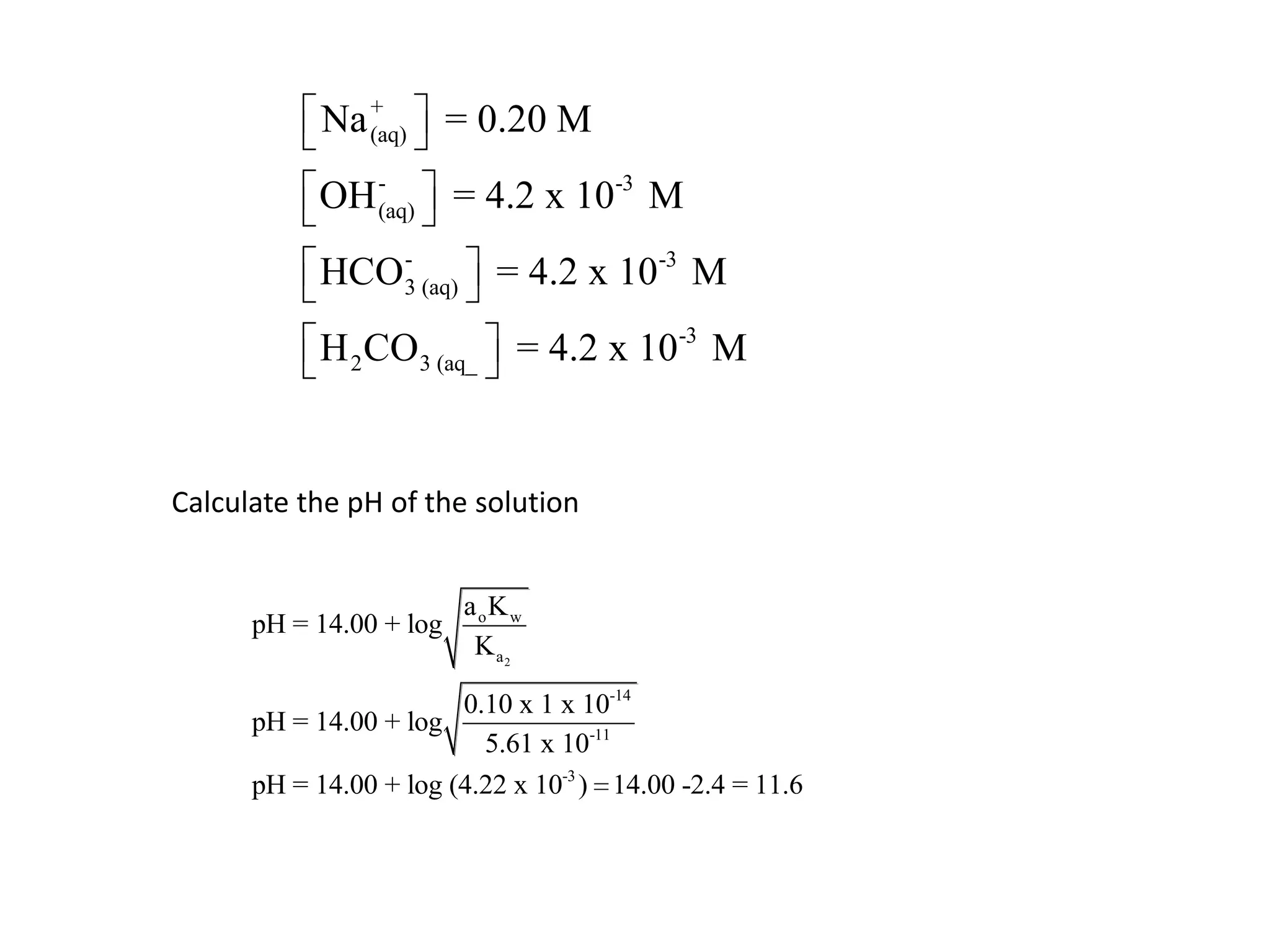
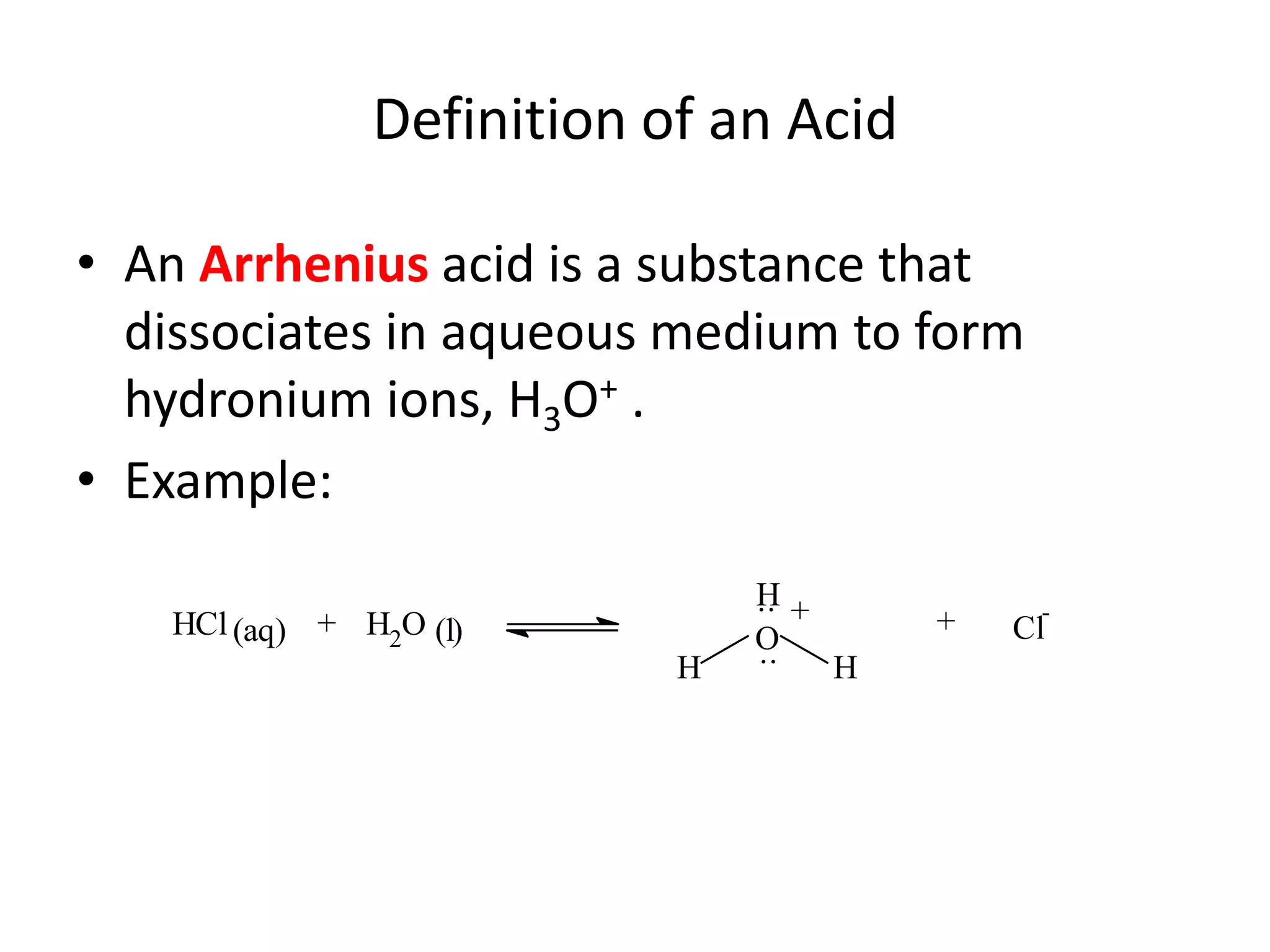
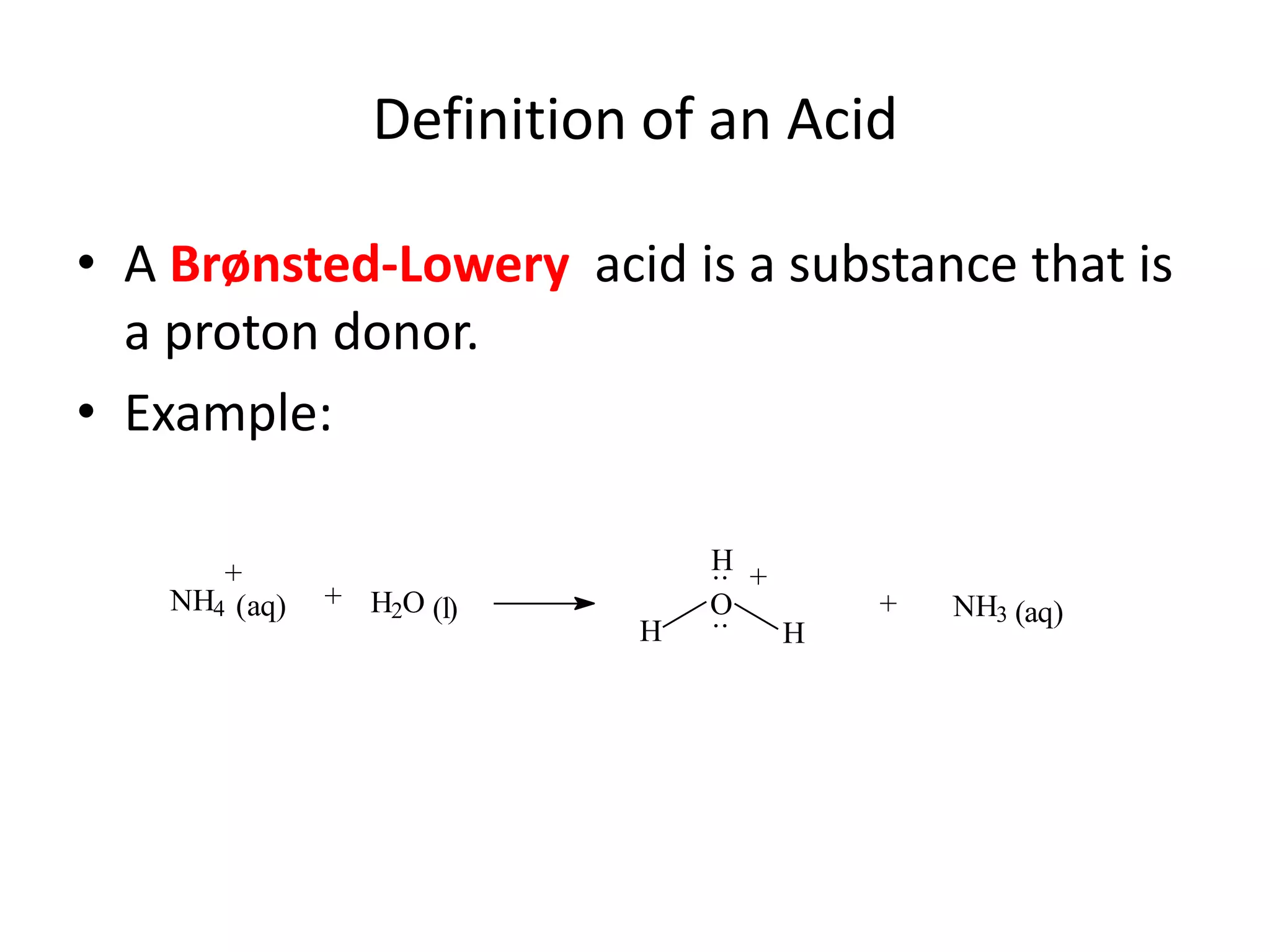
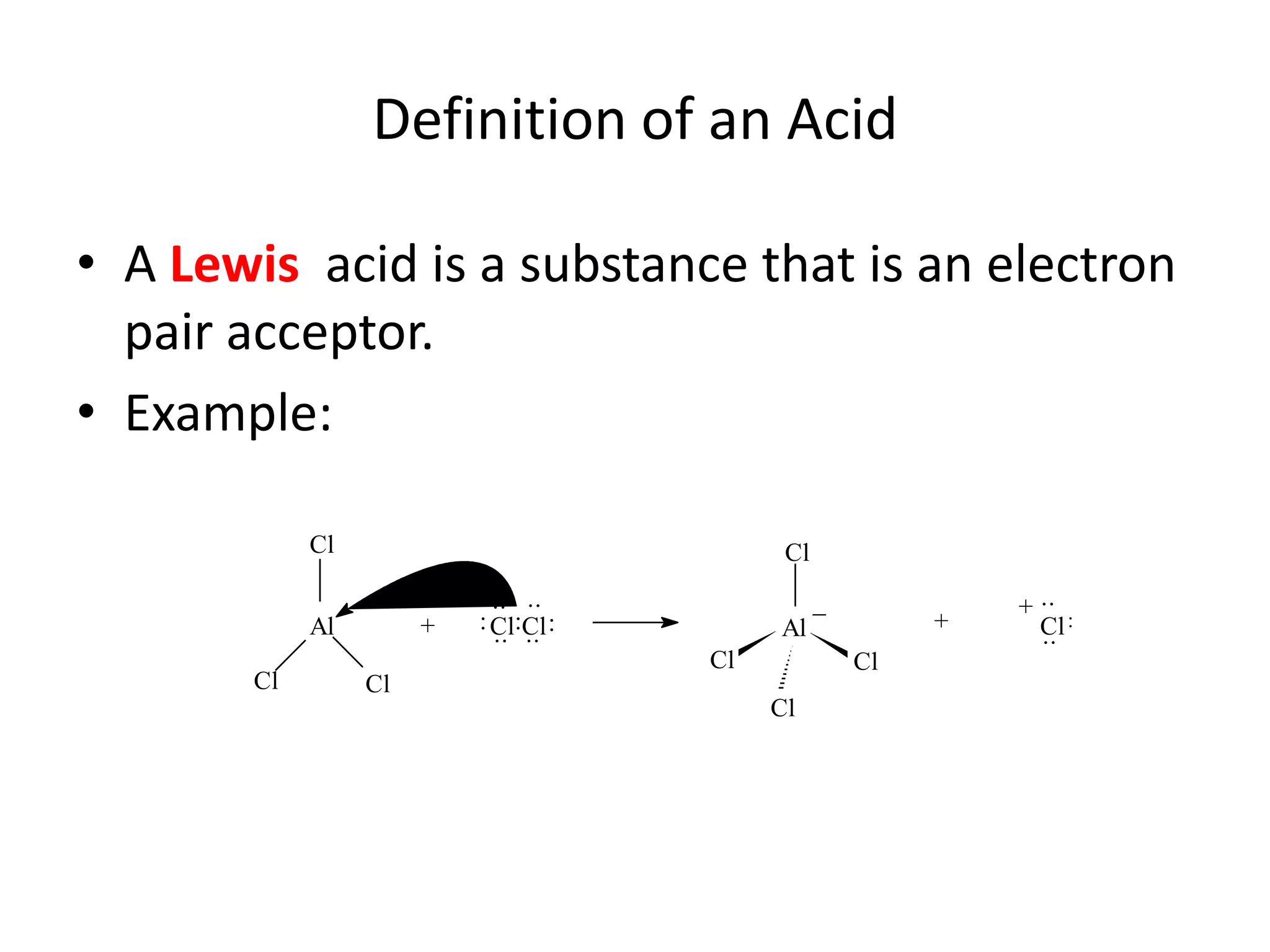
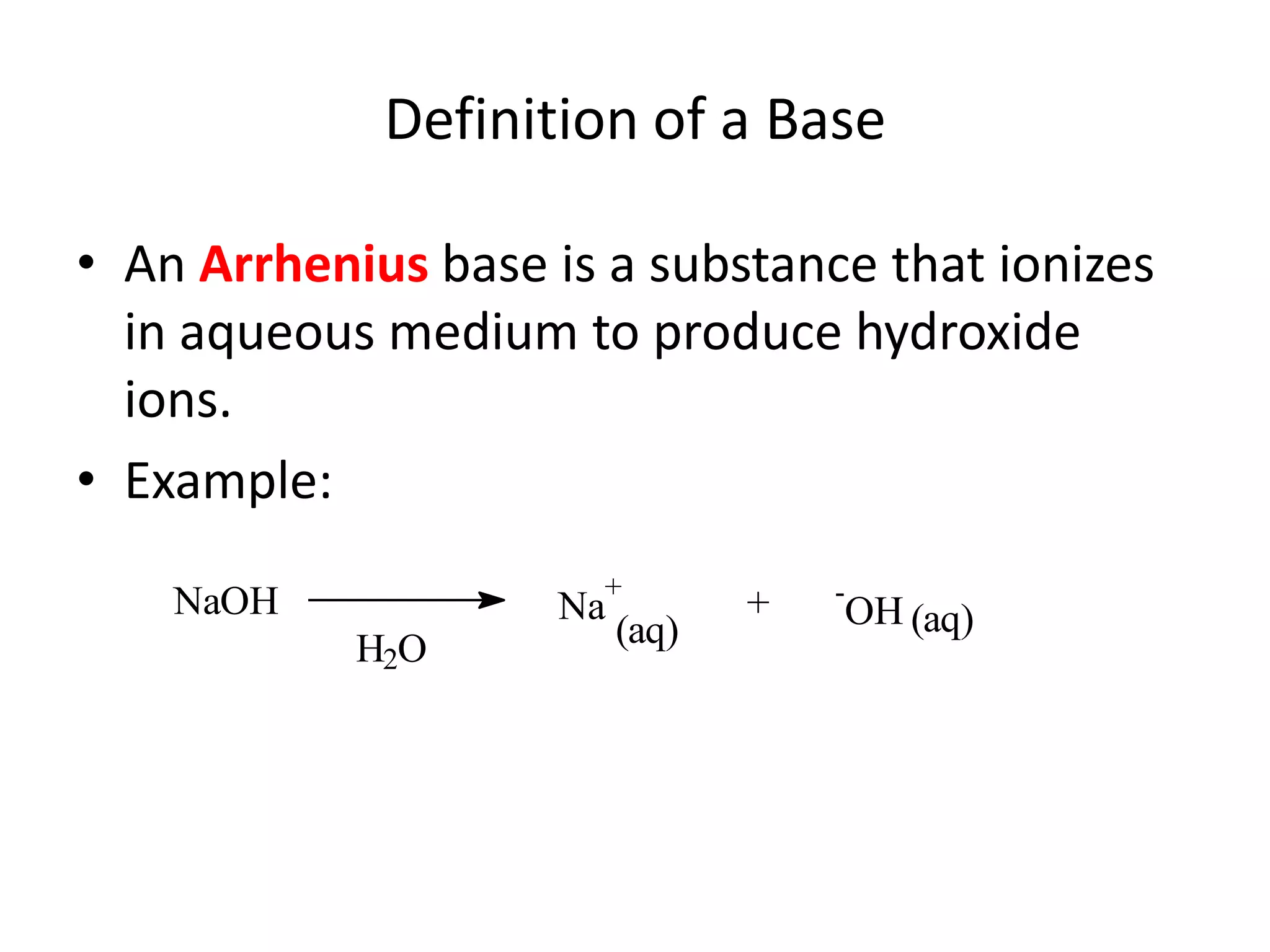
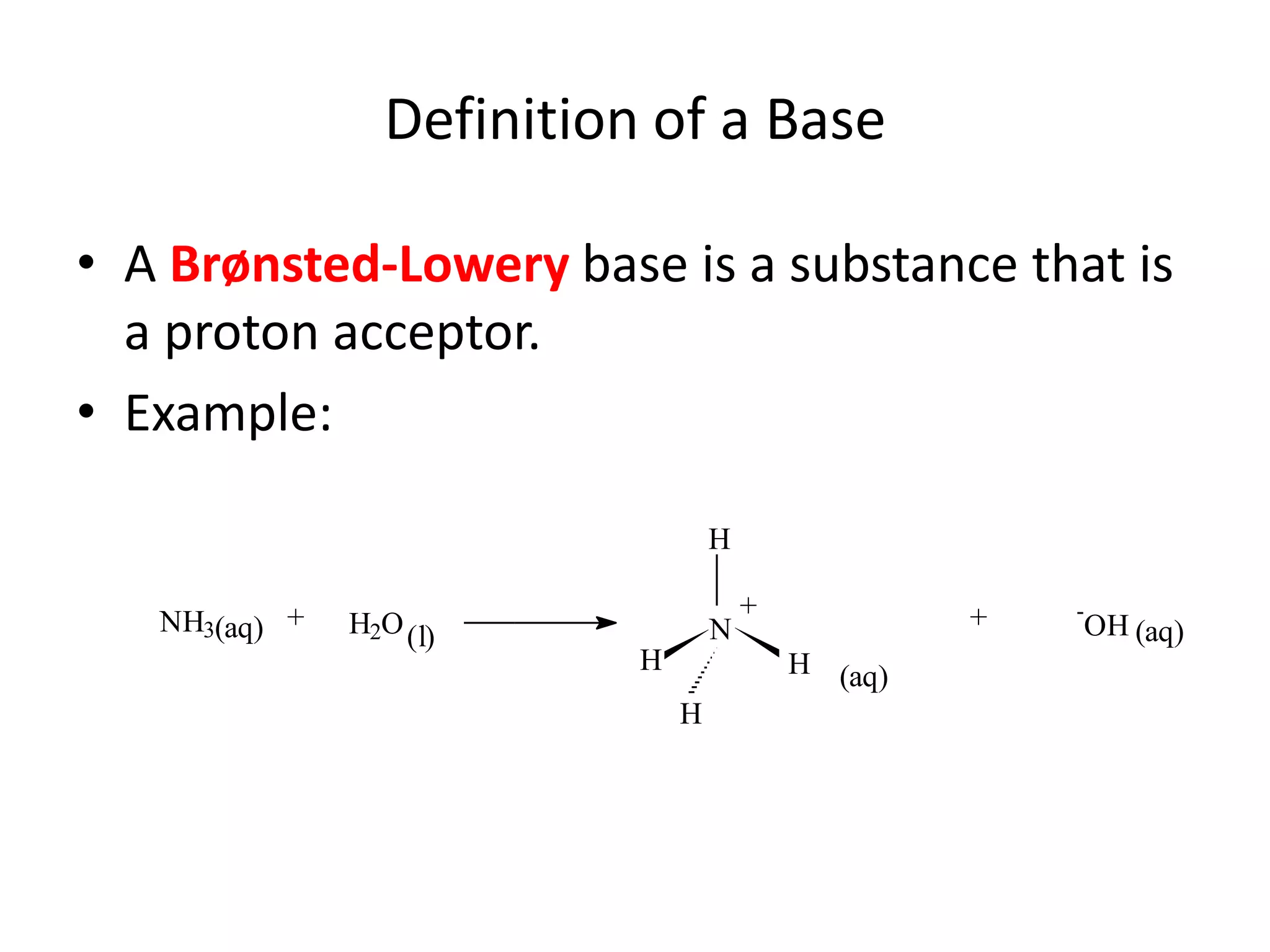
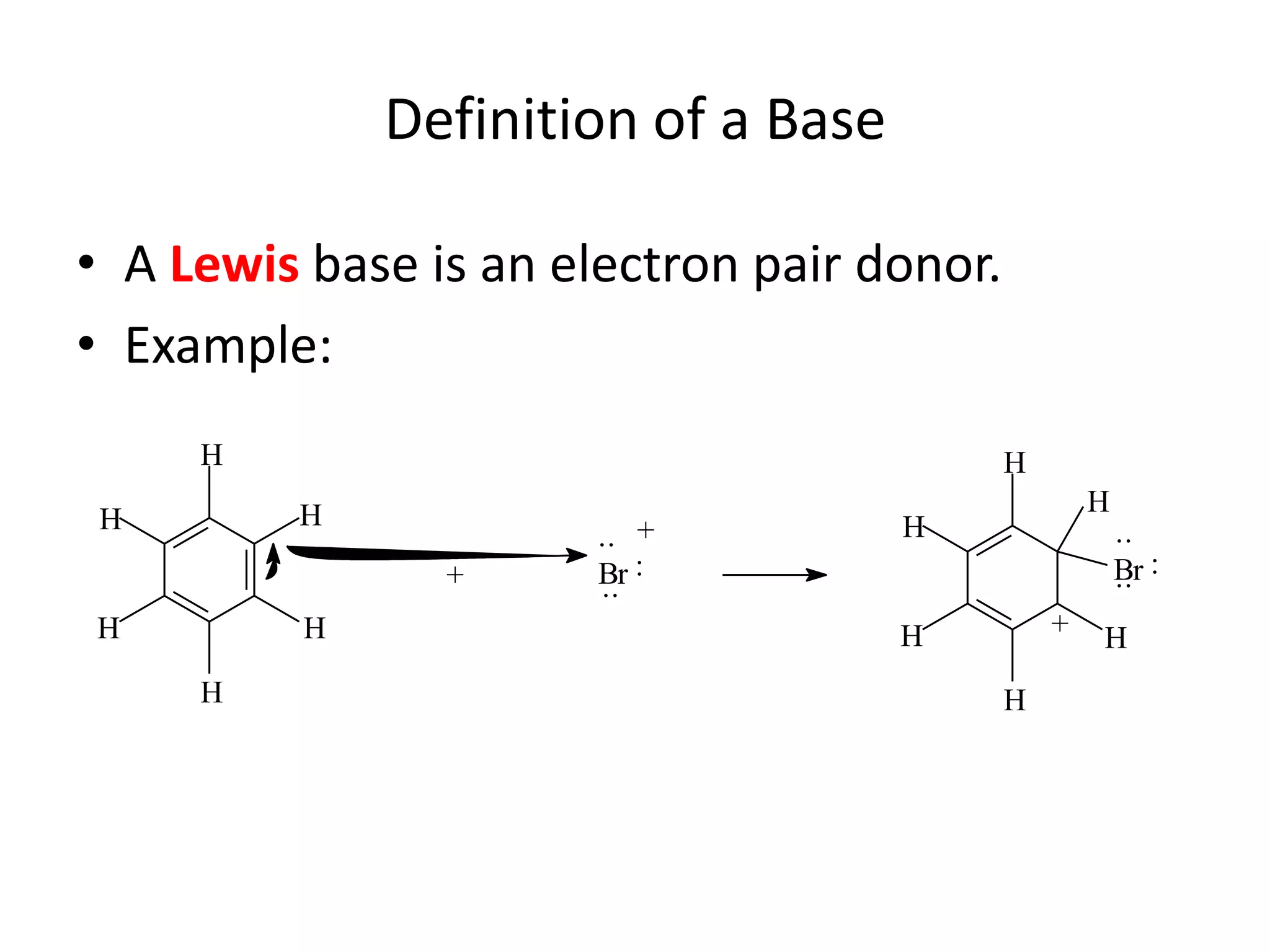
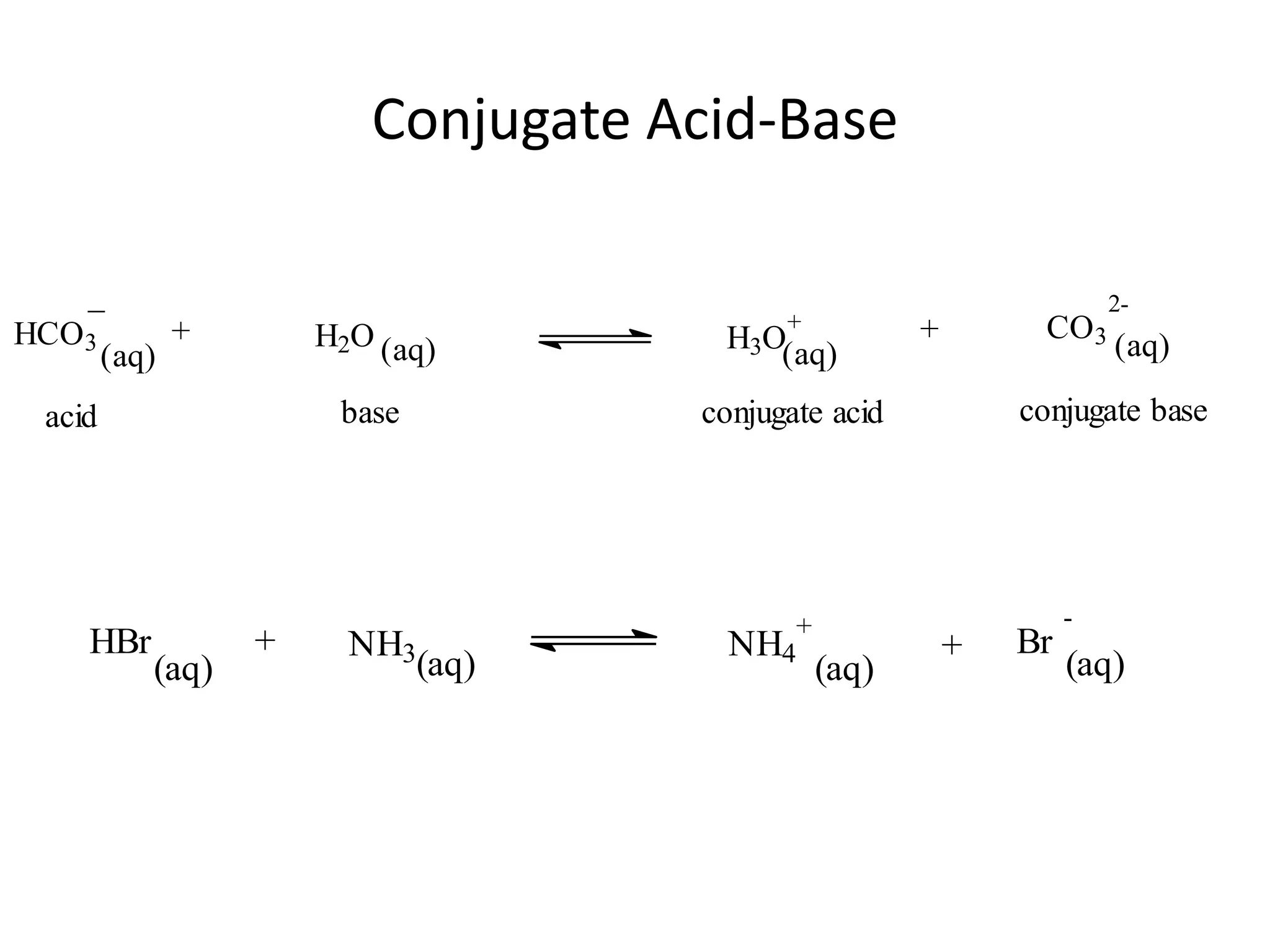
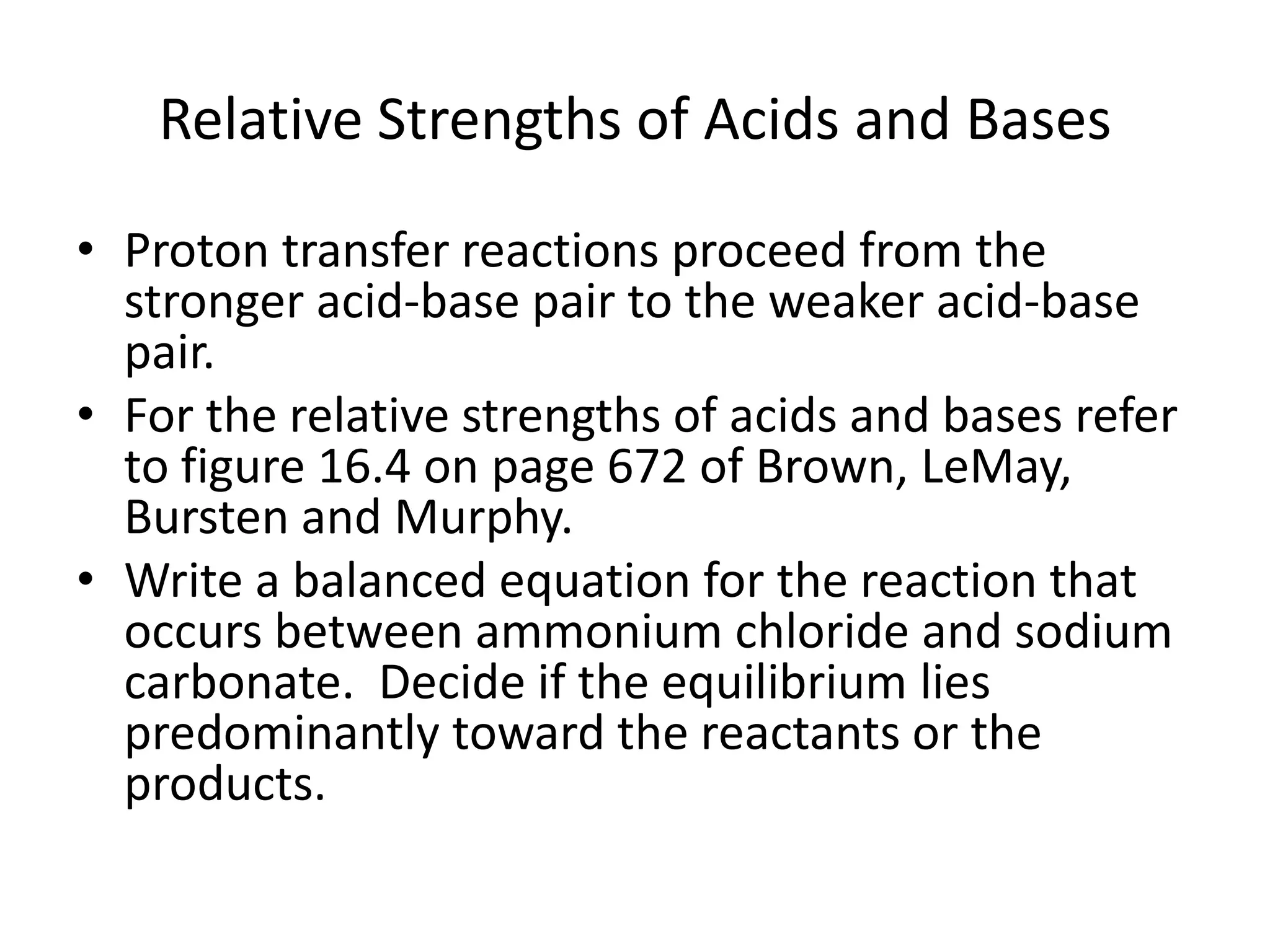
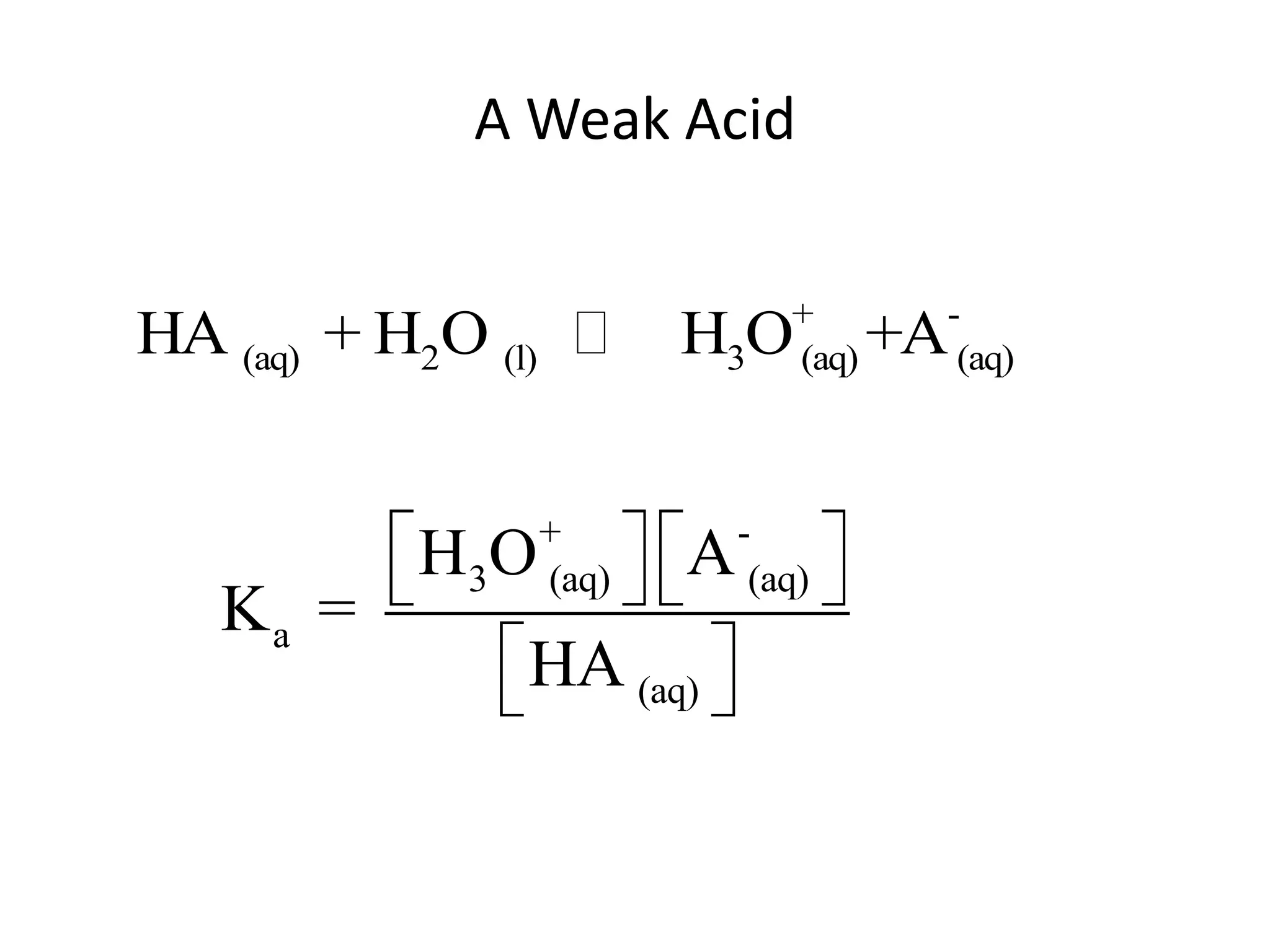
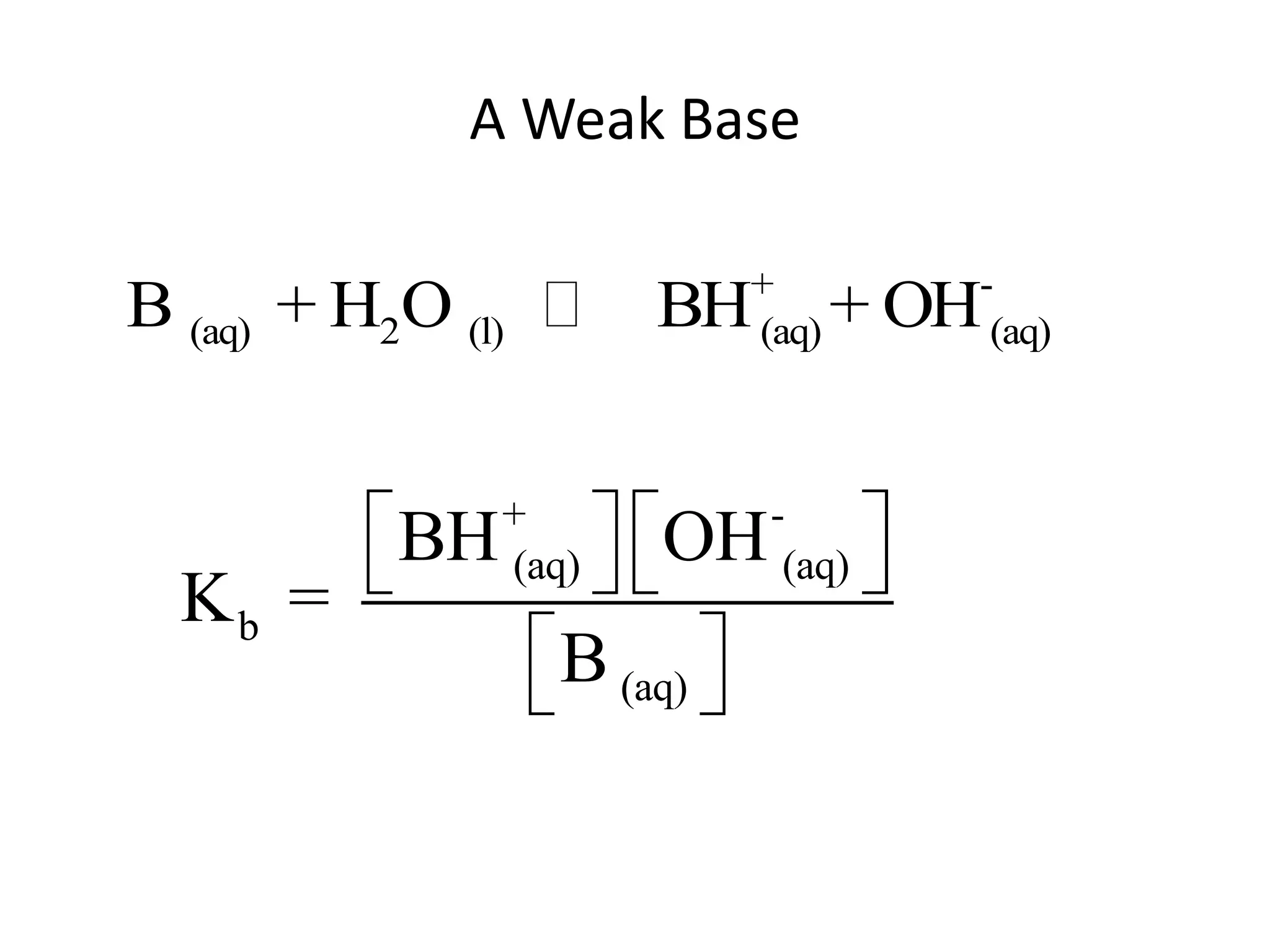
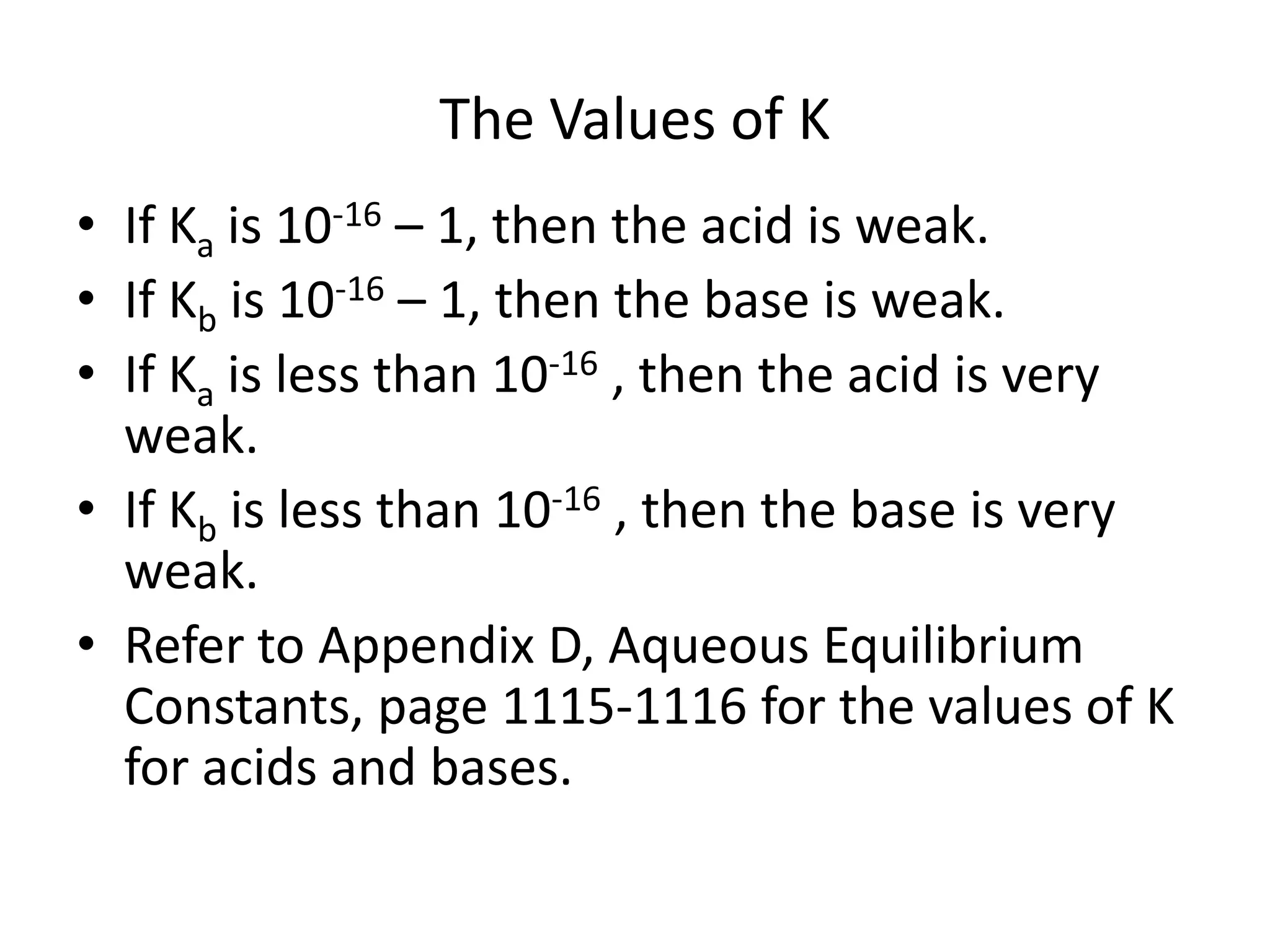
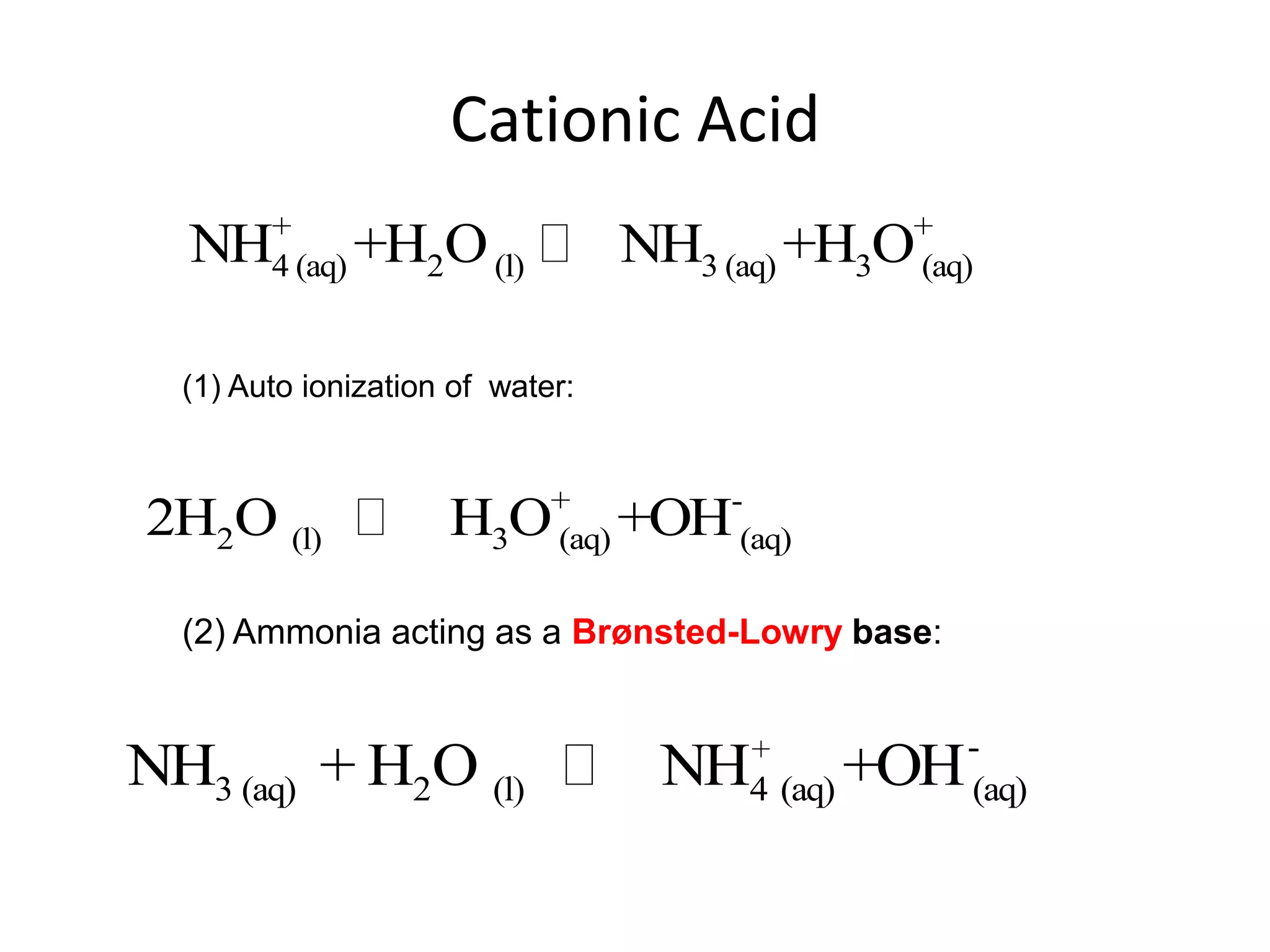
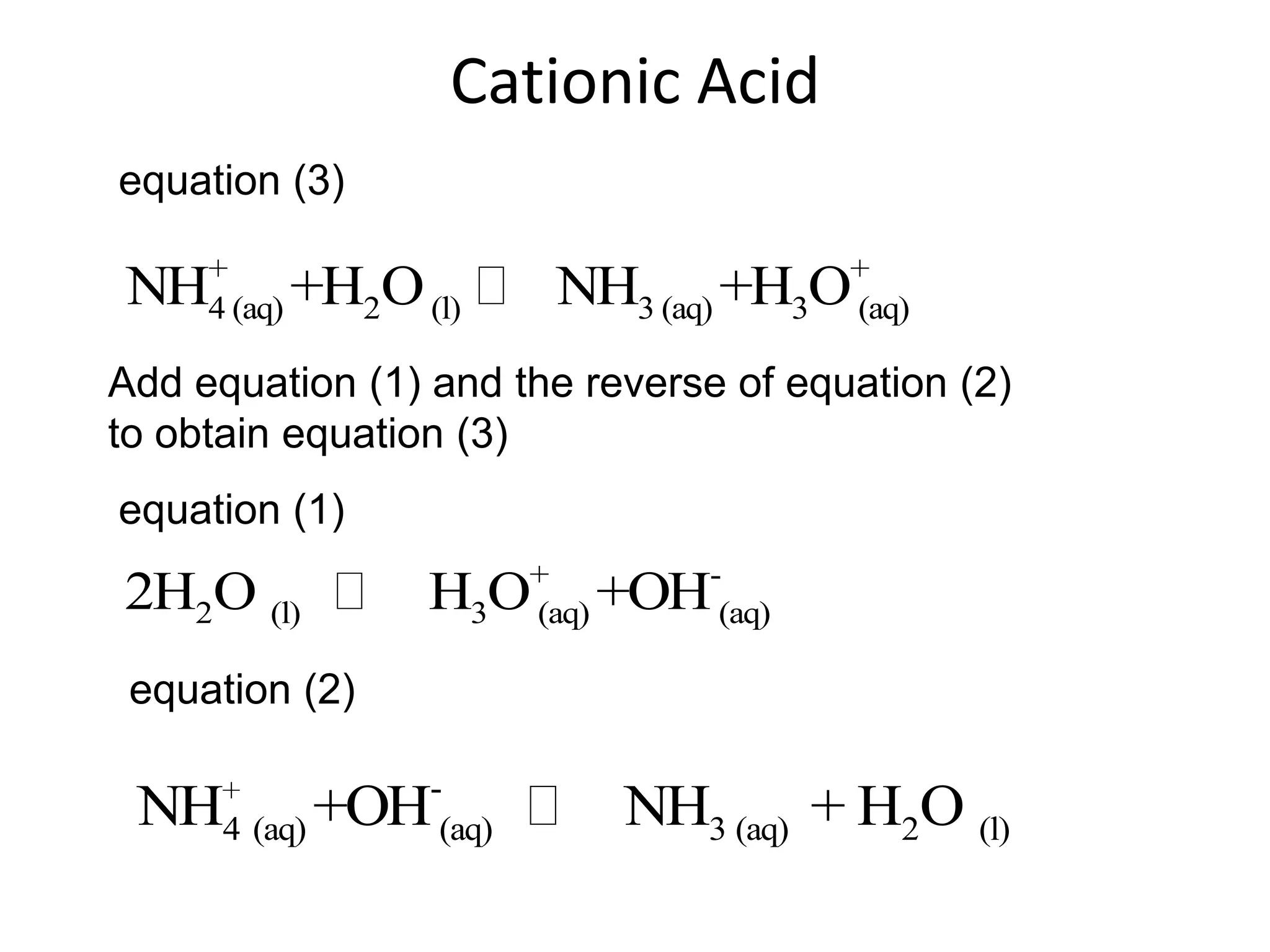
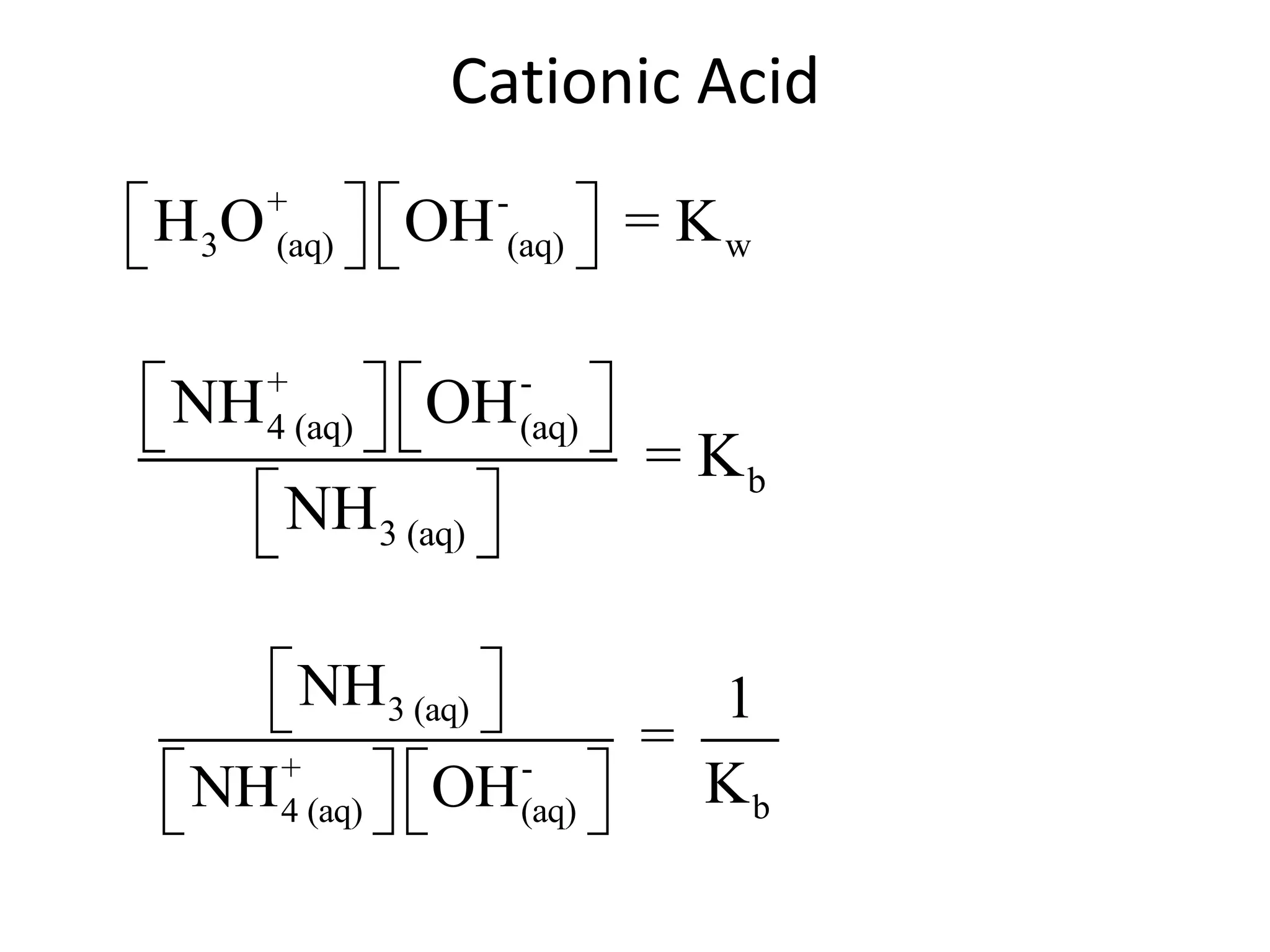
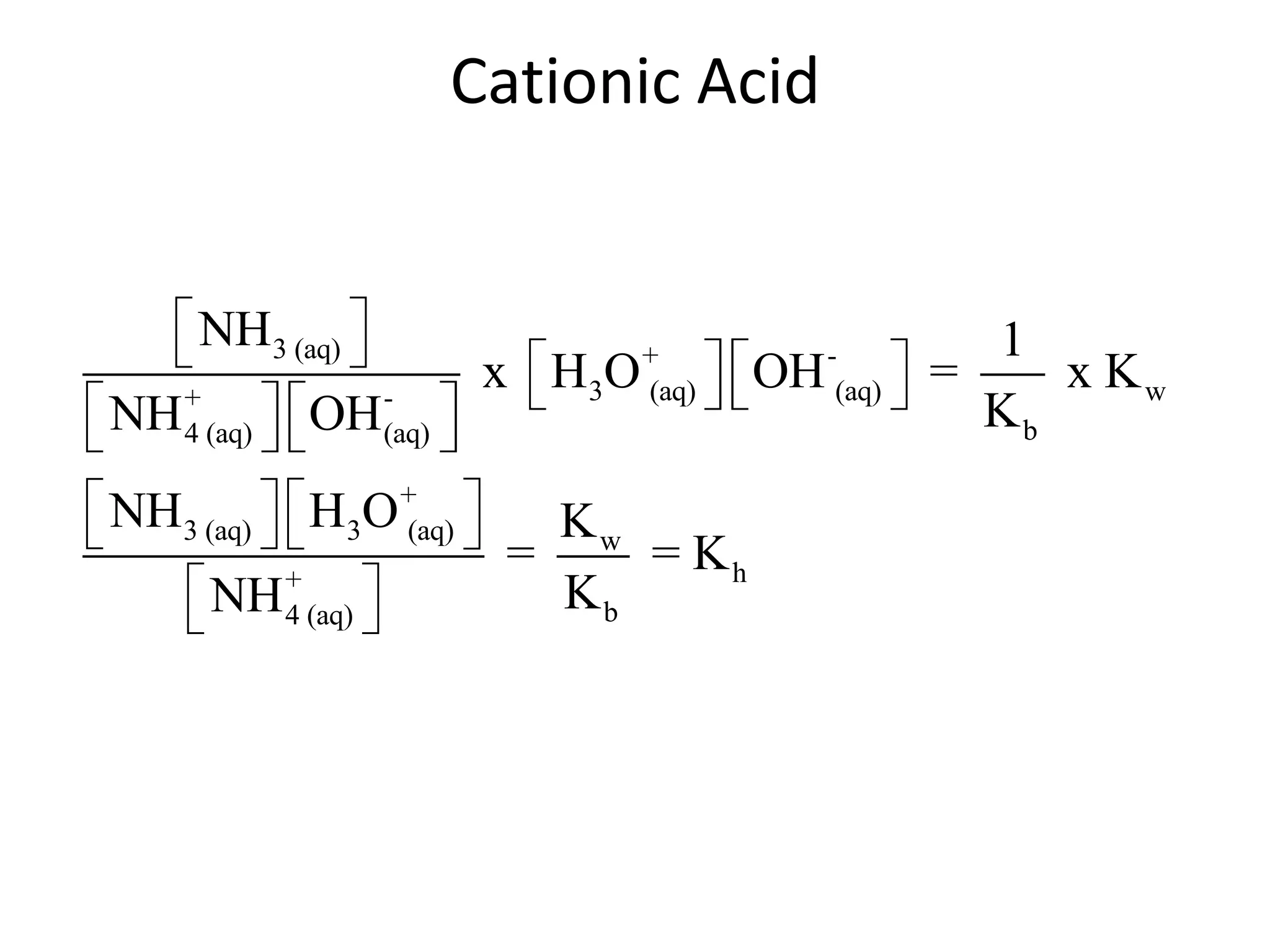
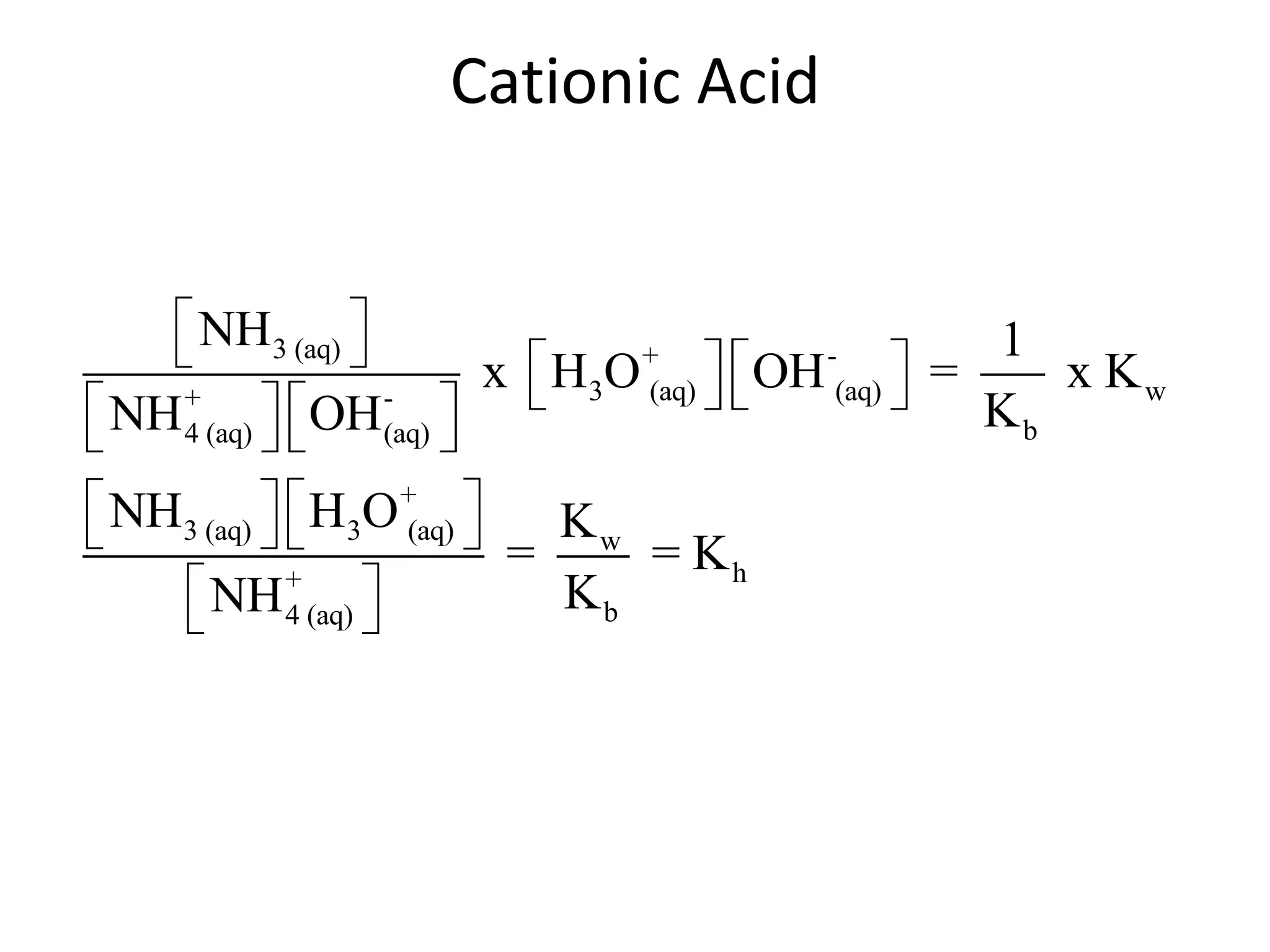
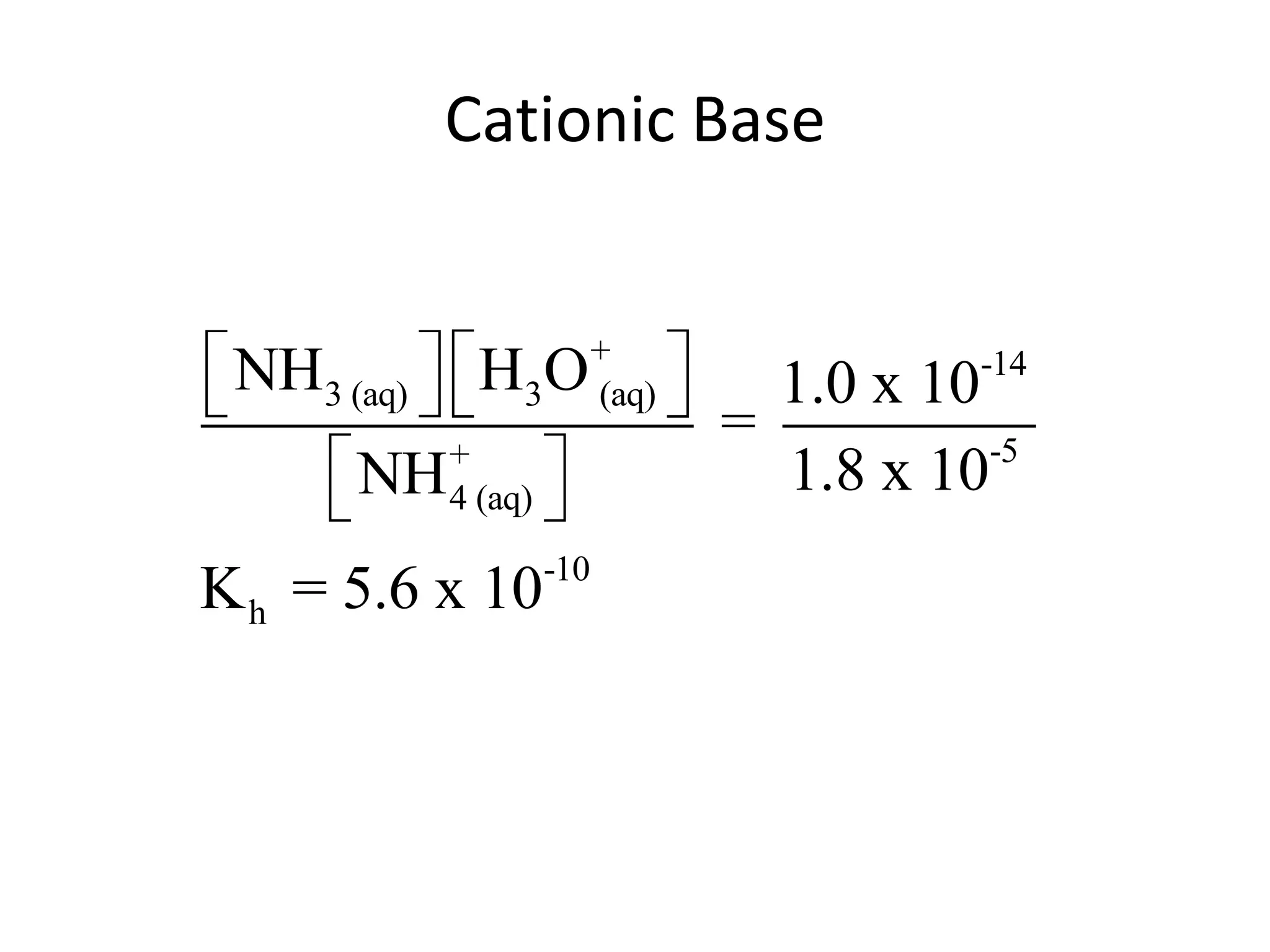
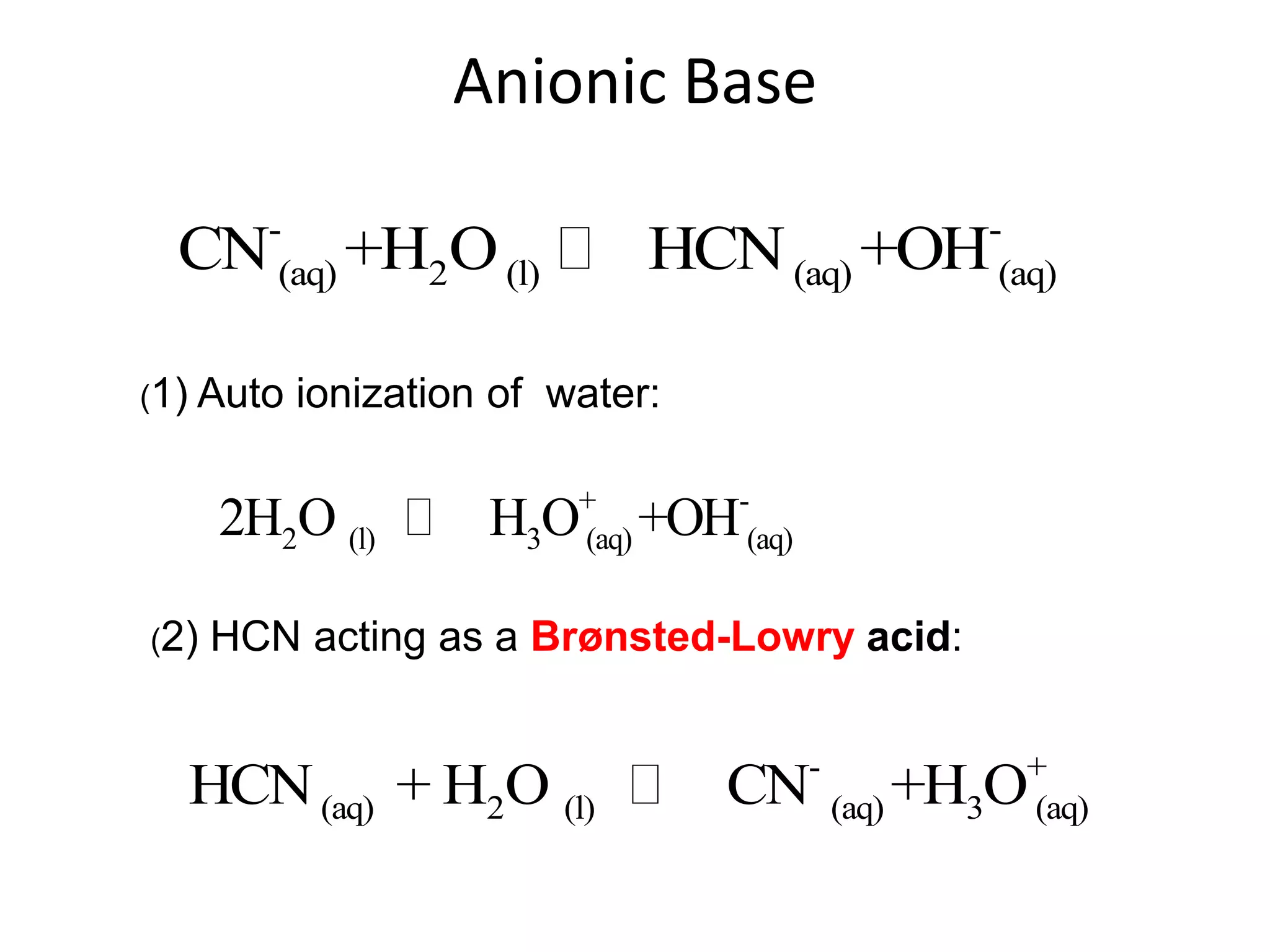
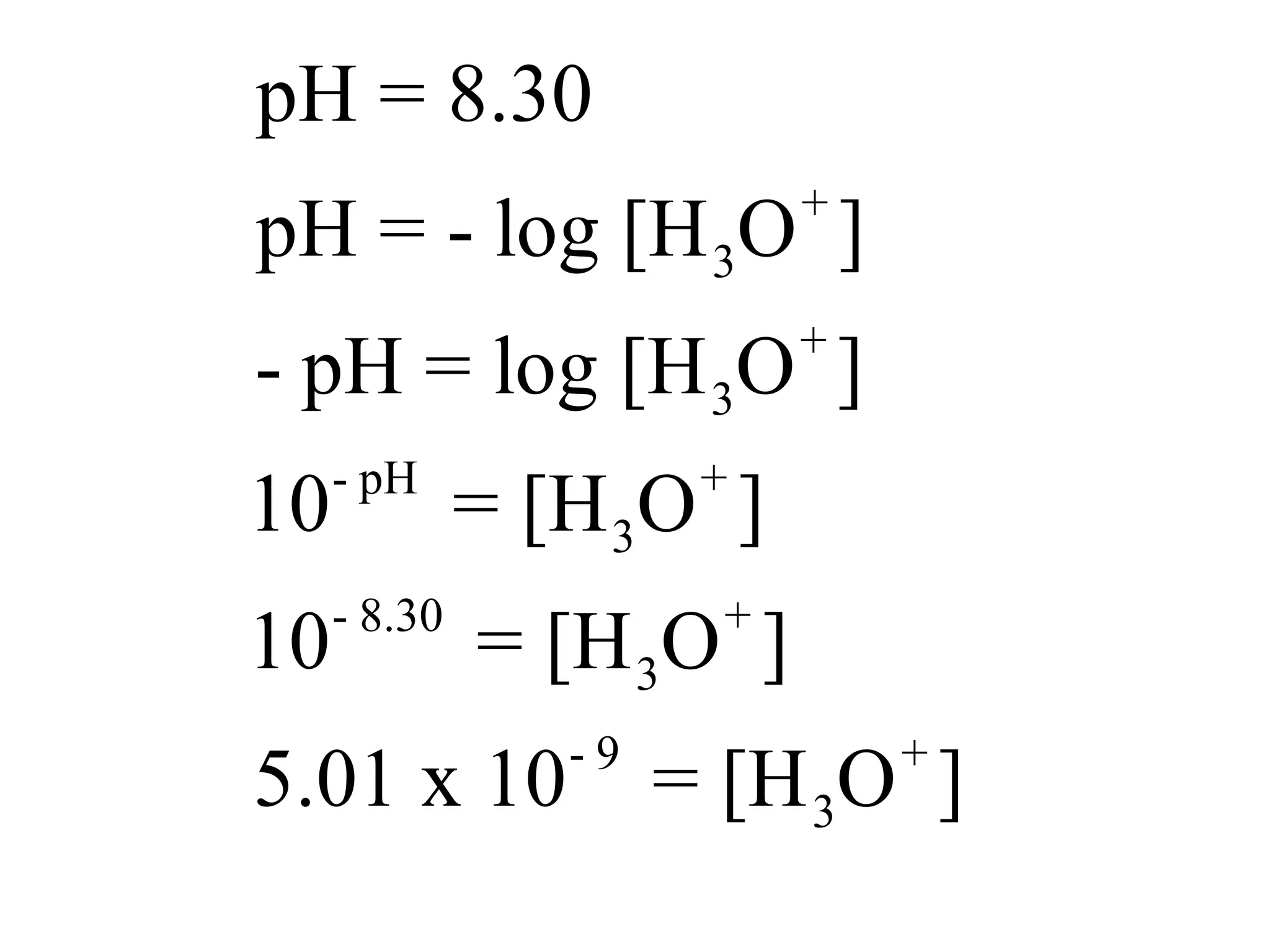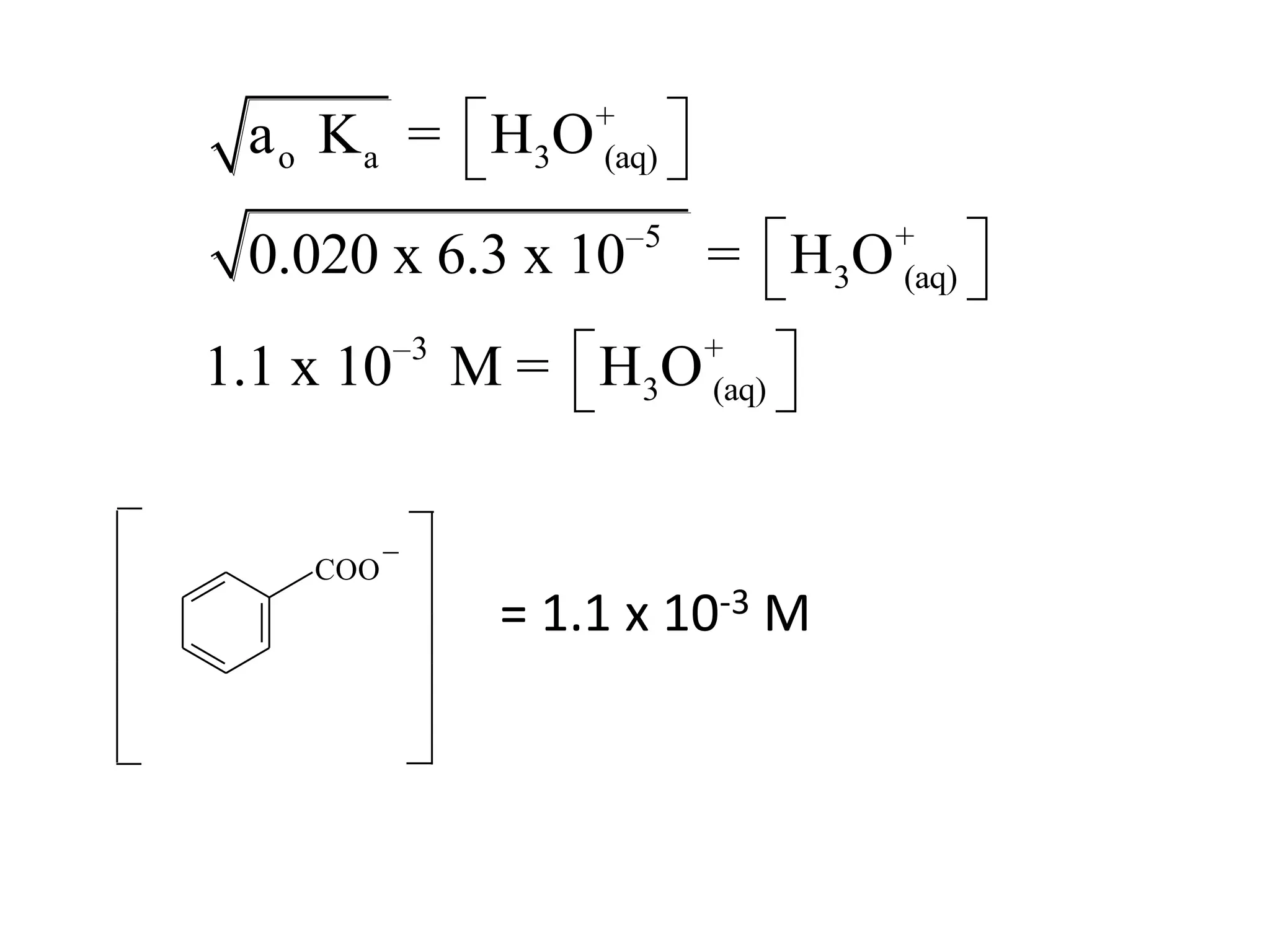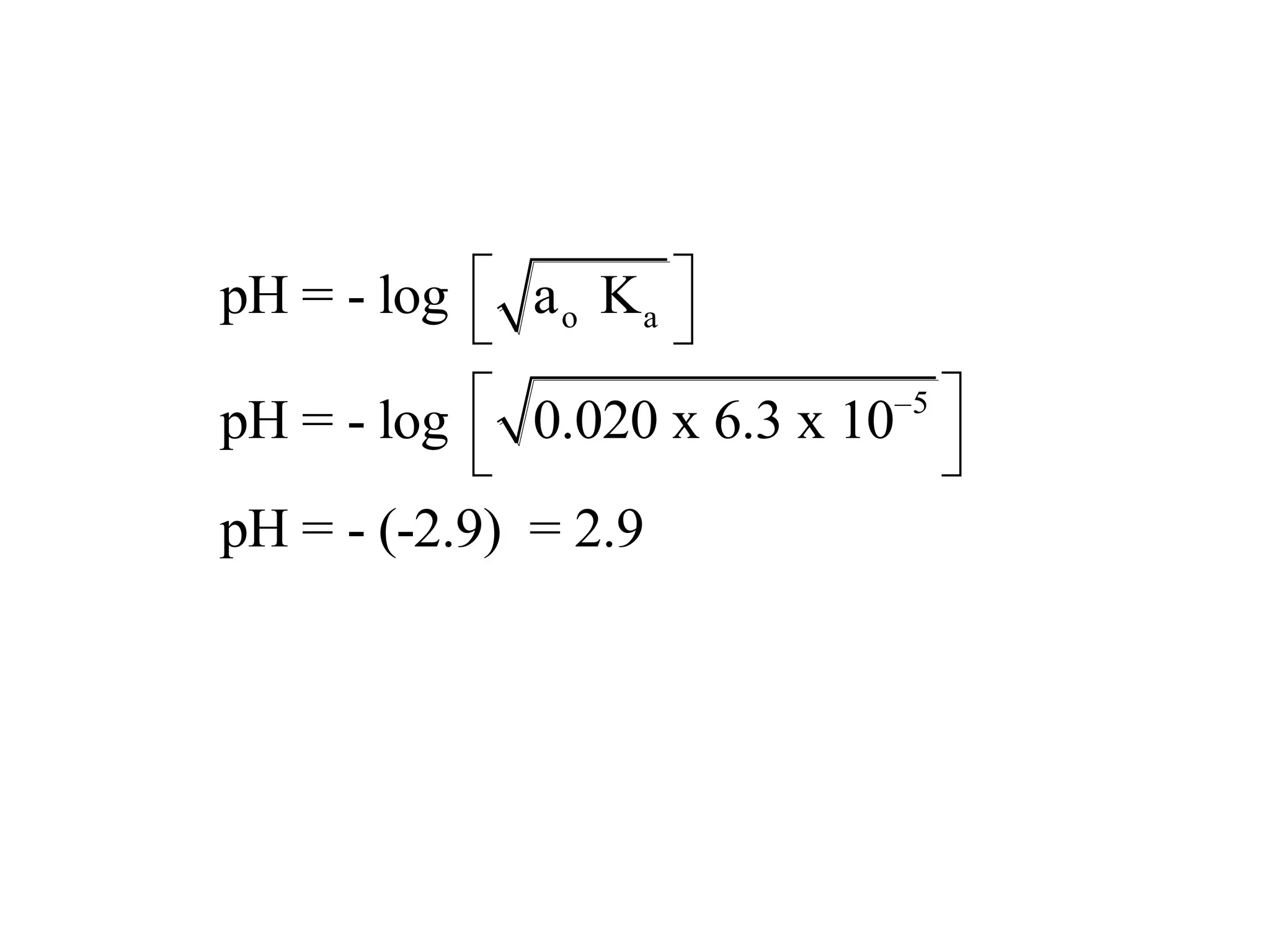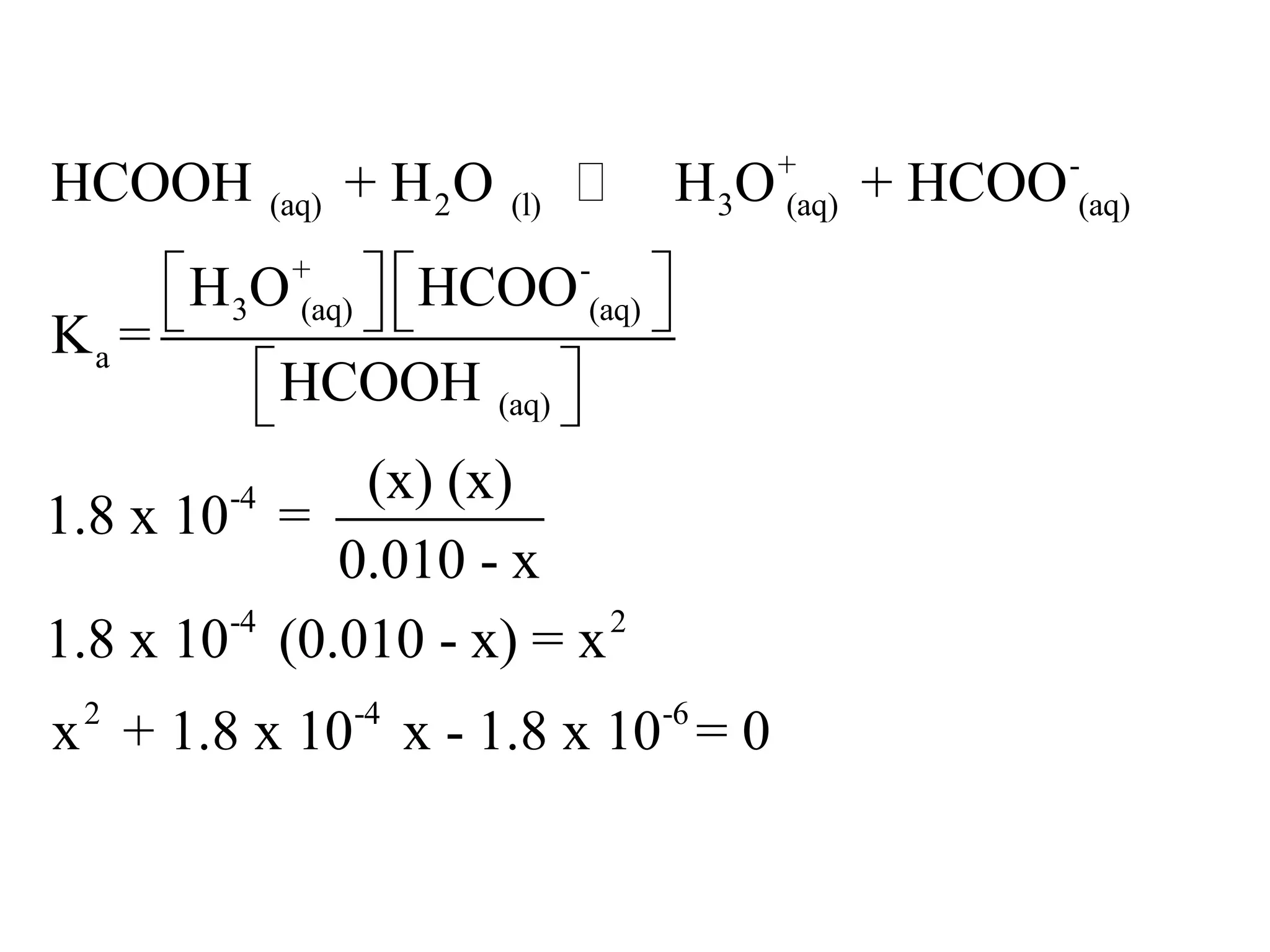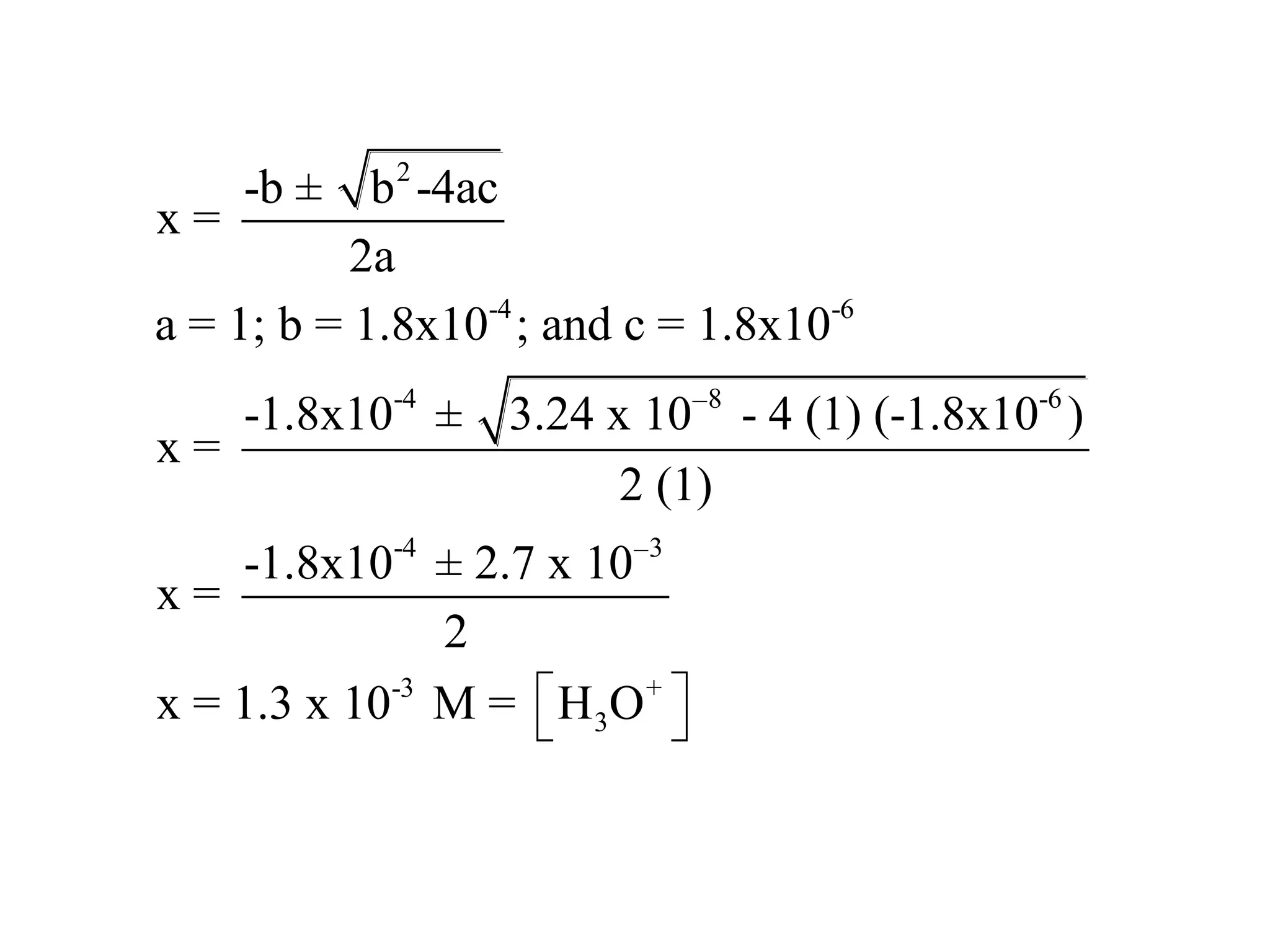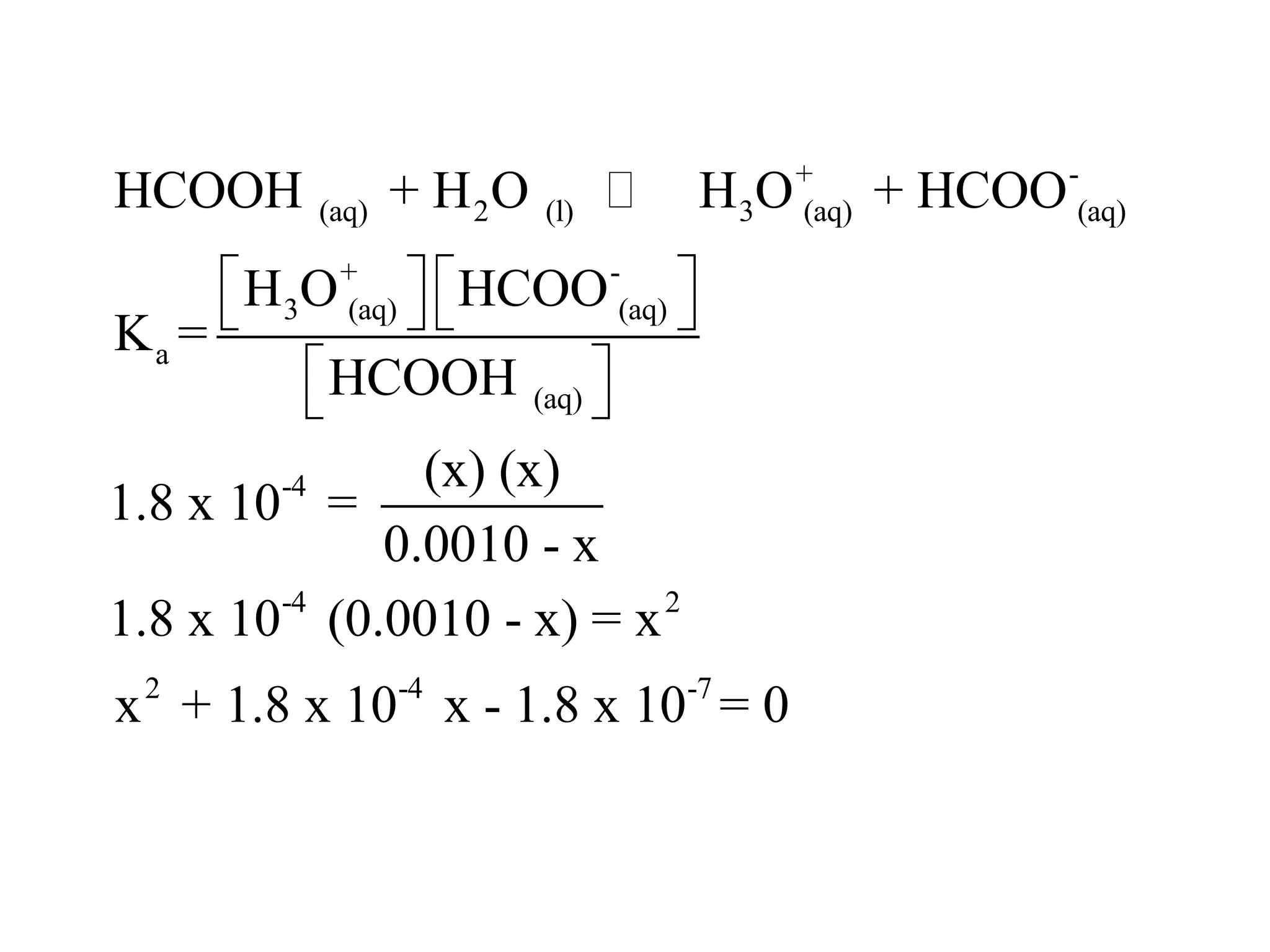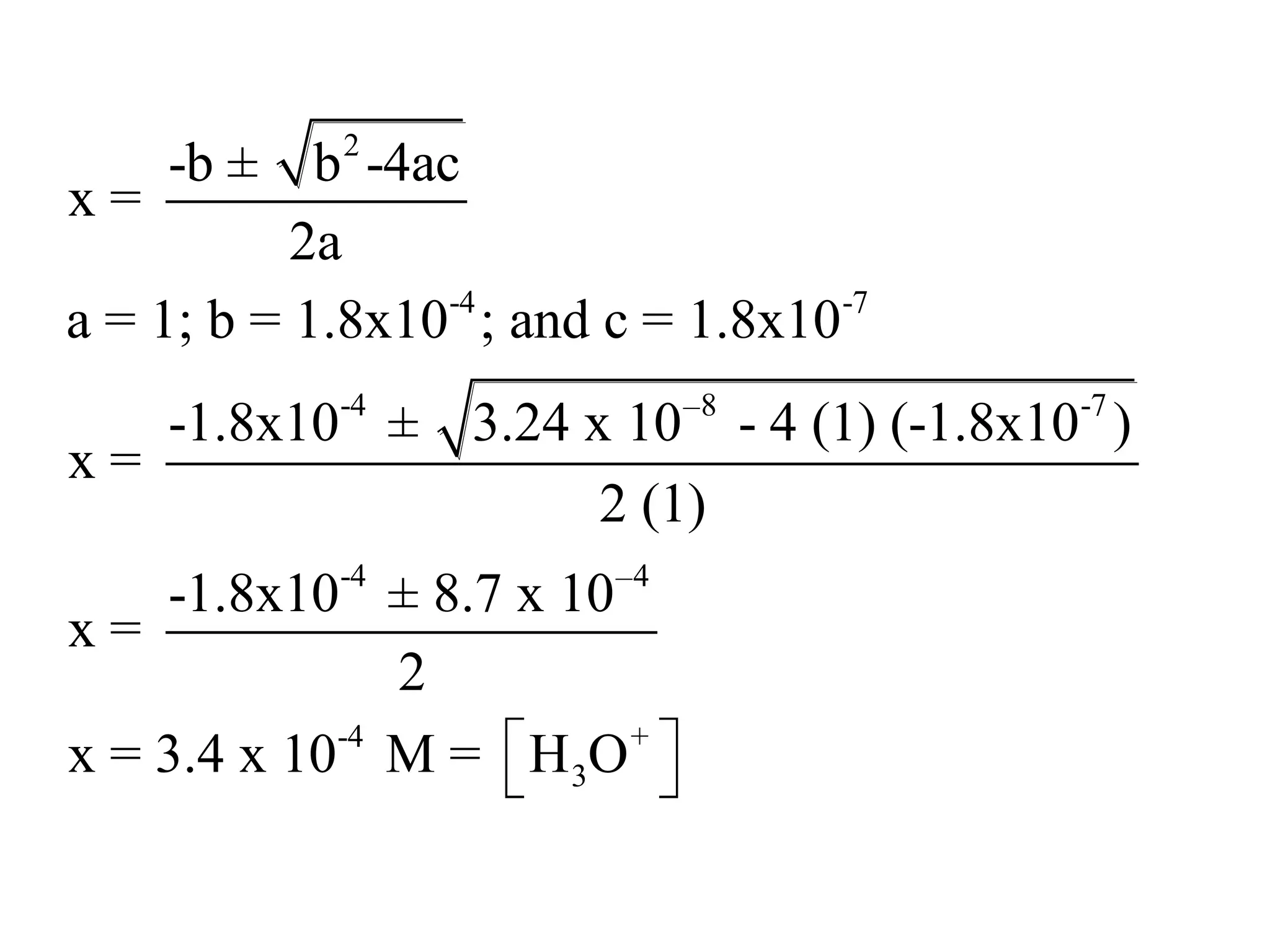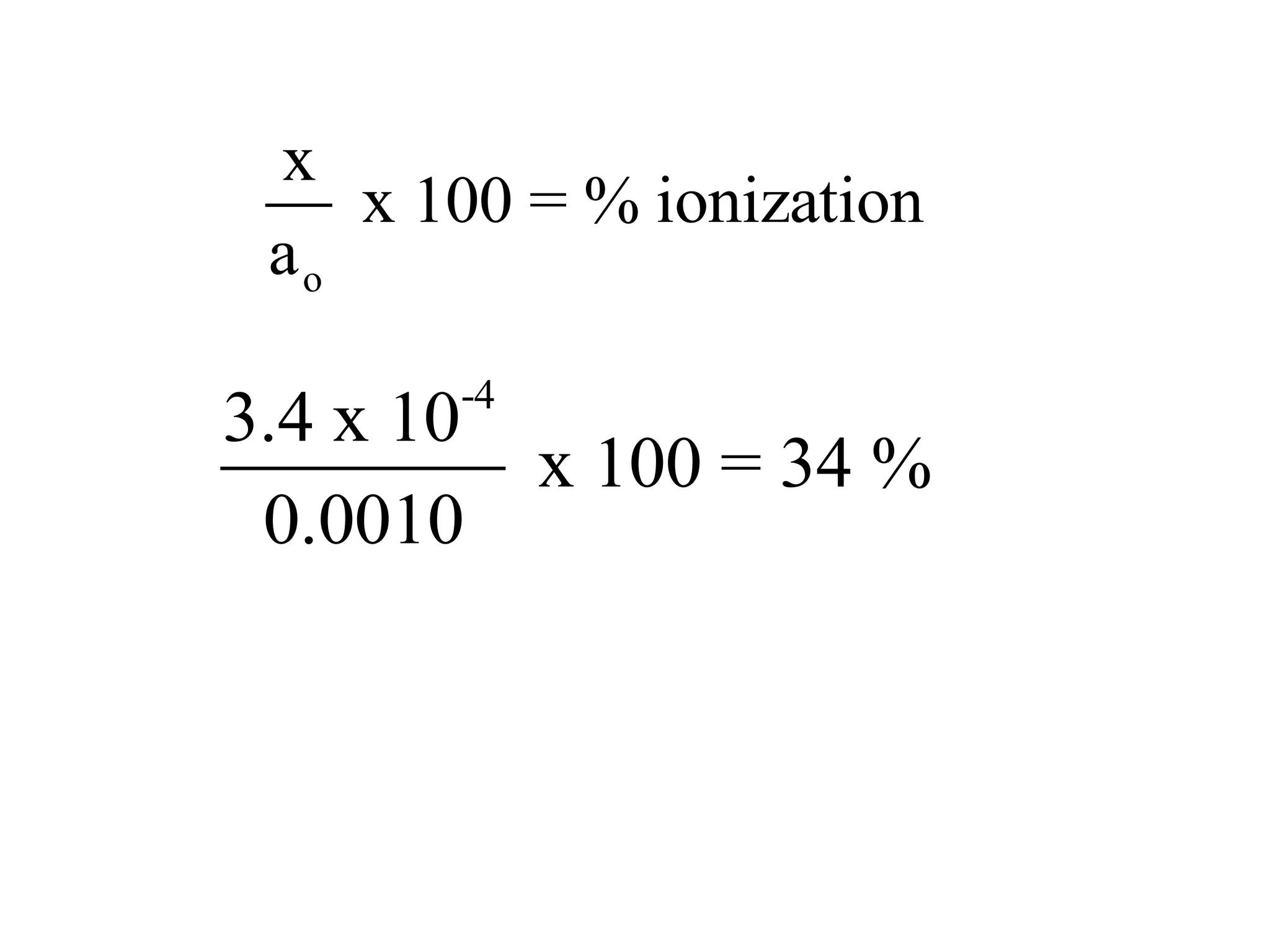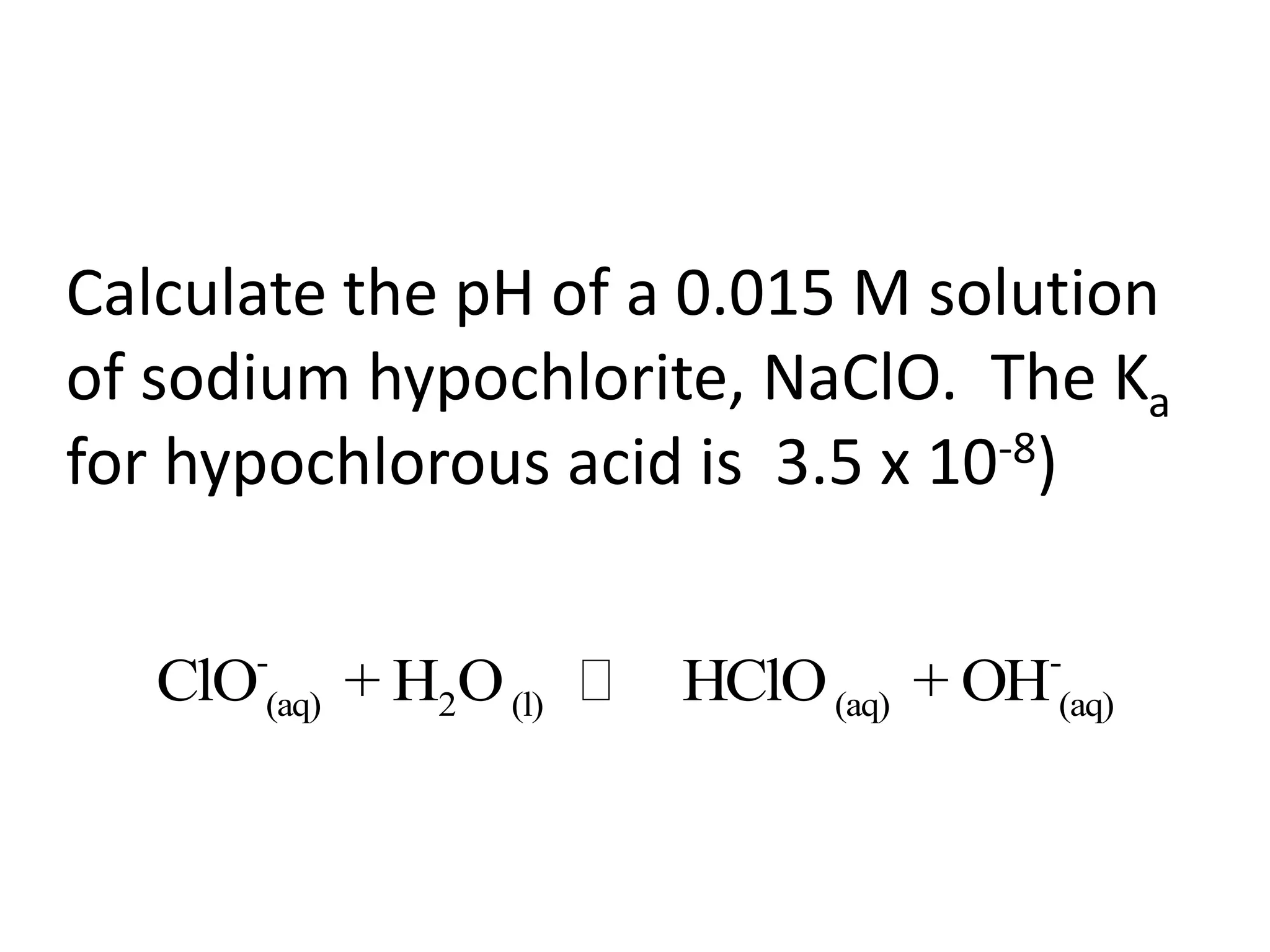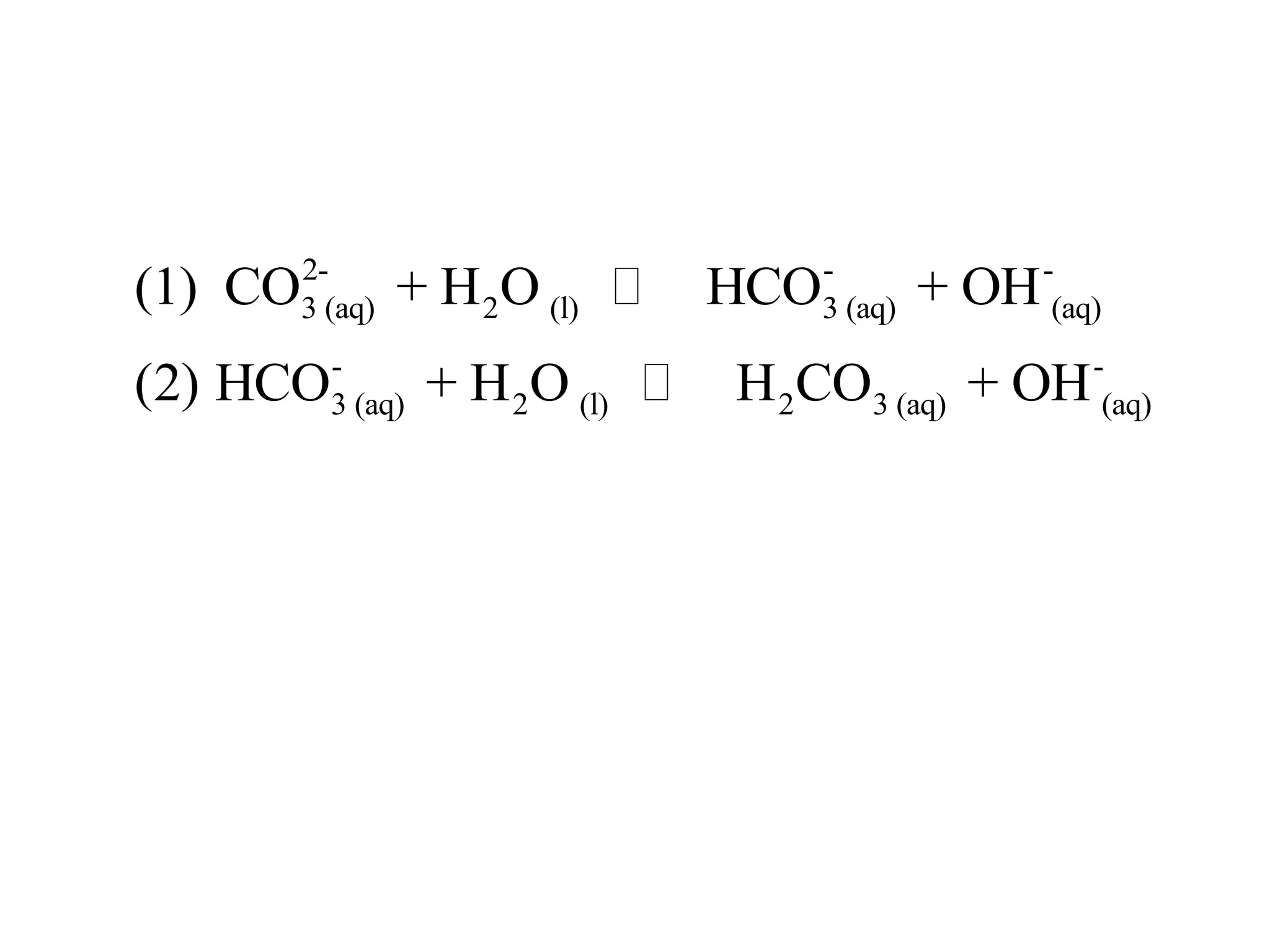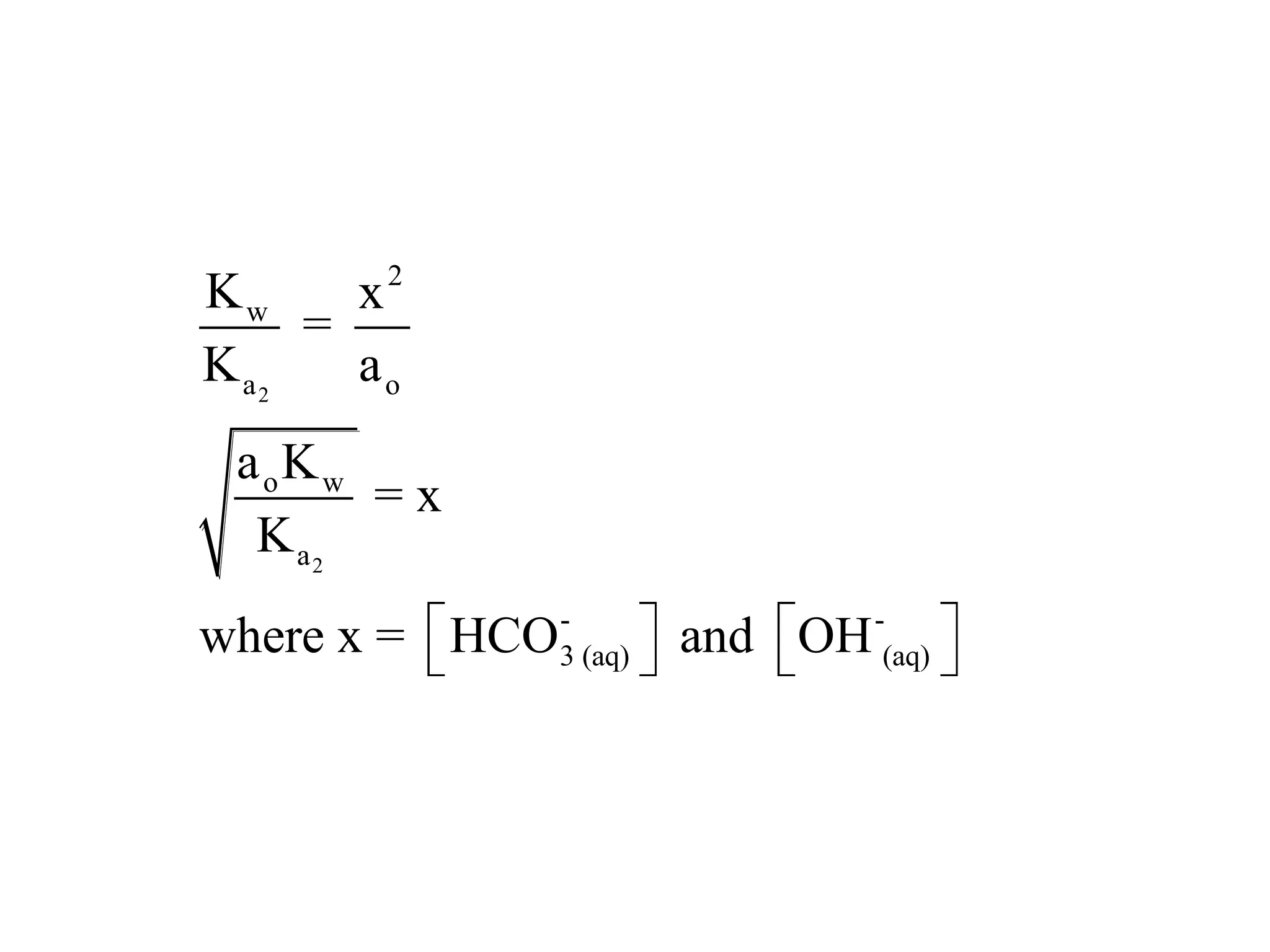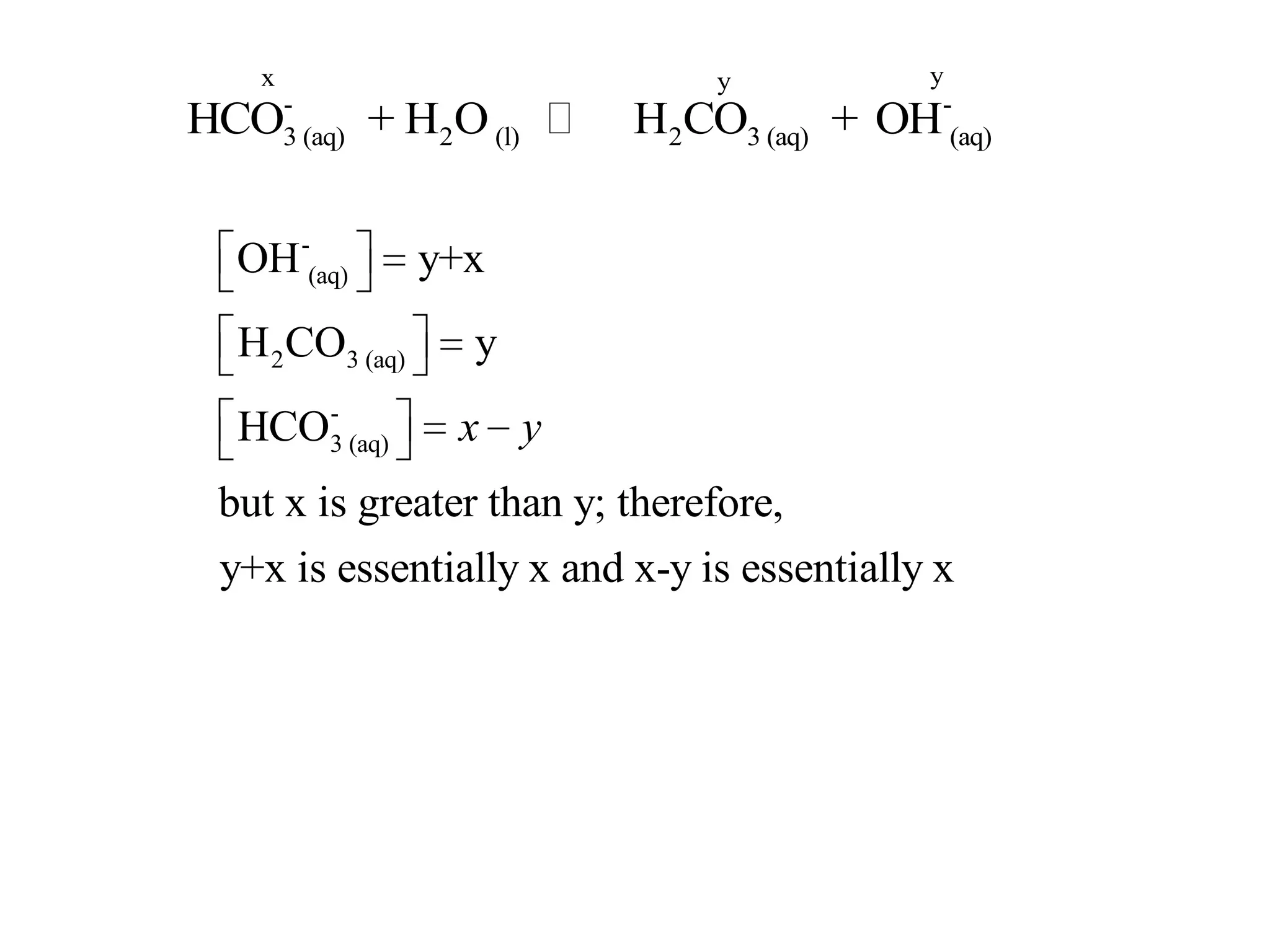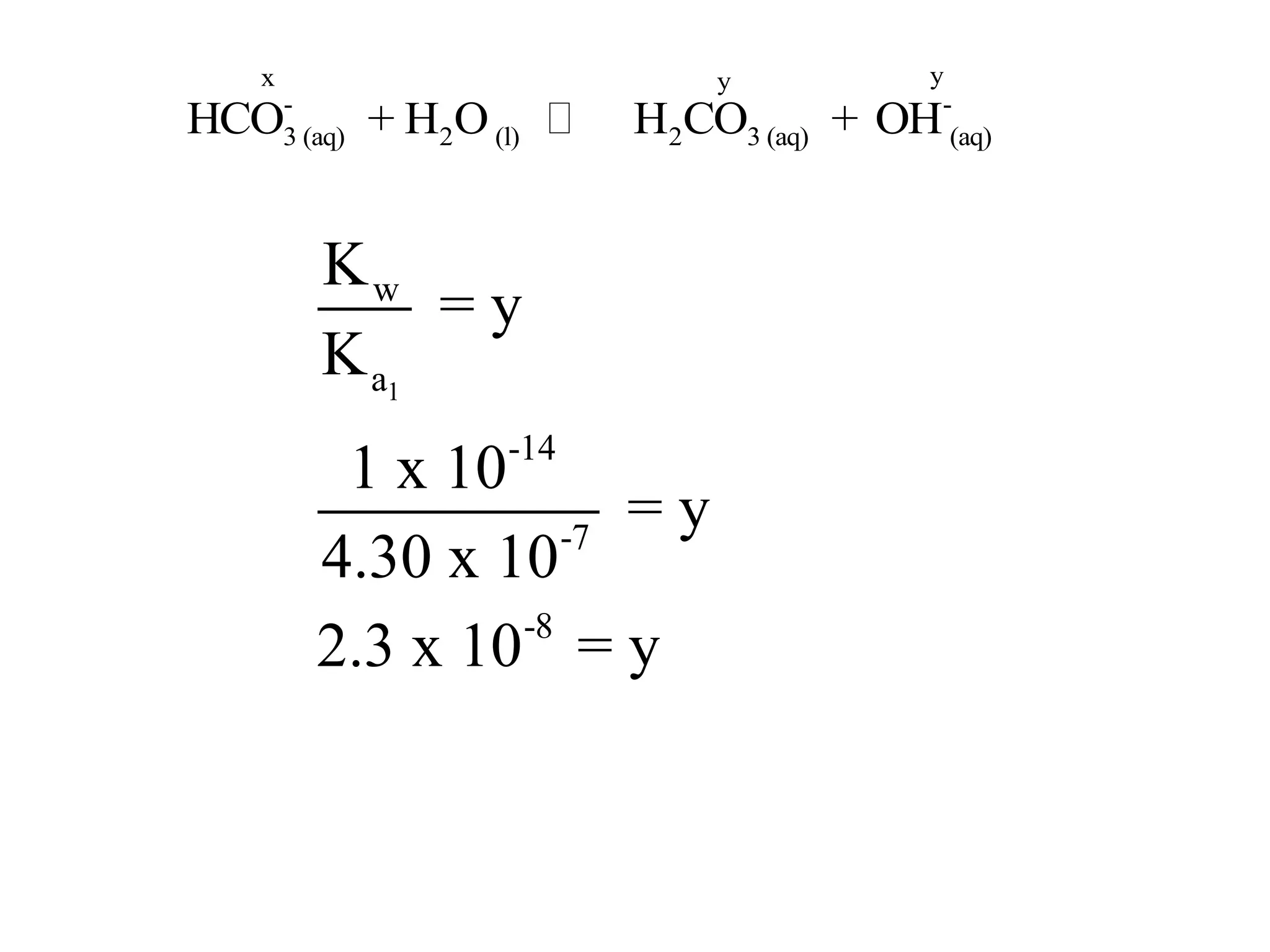The document discusses acid-base equilibrium, defining Arrhenius, Brønsted-Lowry, and Lewis acids and bases, along with the relationships between their strengths and corresponding equilibrium constants (K). It includes examples of calculating pH and the dissociation constants (Ka and Kb) for various acids and bases, alongside applications for calculating equilibrium concentrations and pH in different solutions. The content also covers specific cases, such as weak acids and bases, auto ionization of water, and the common ion effect.


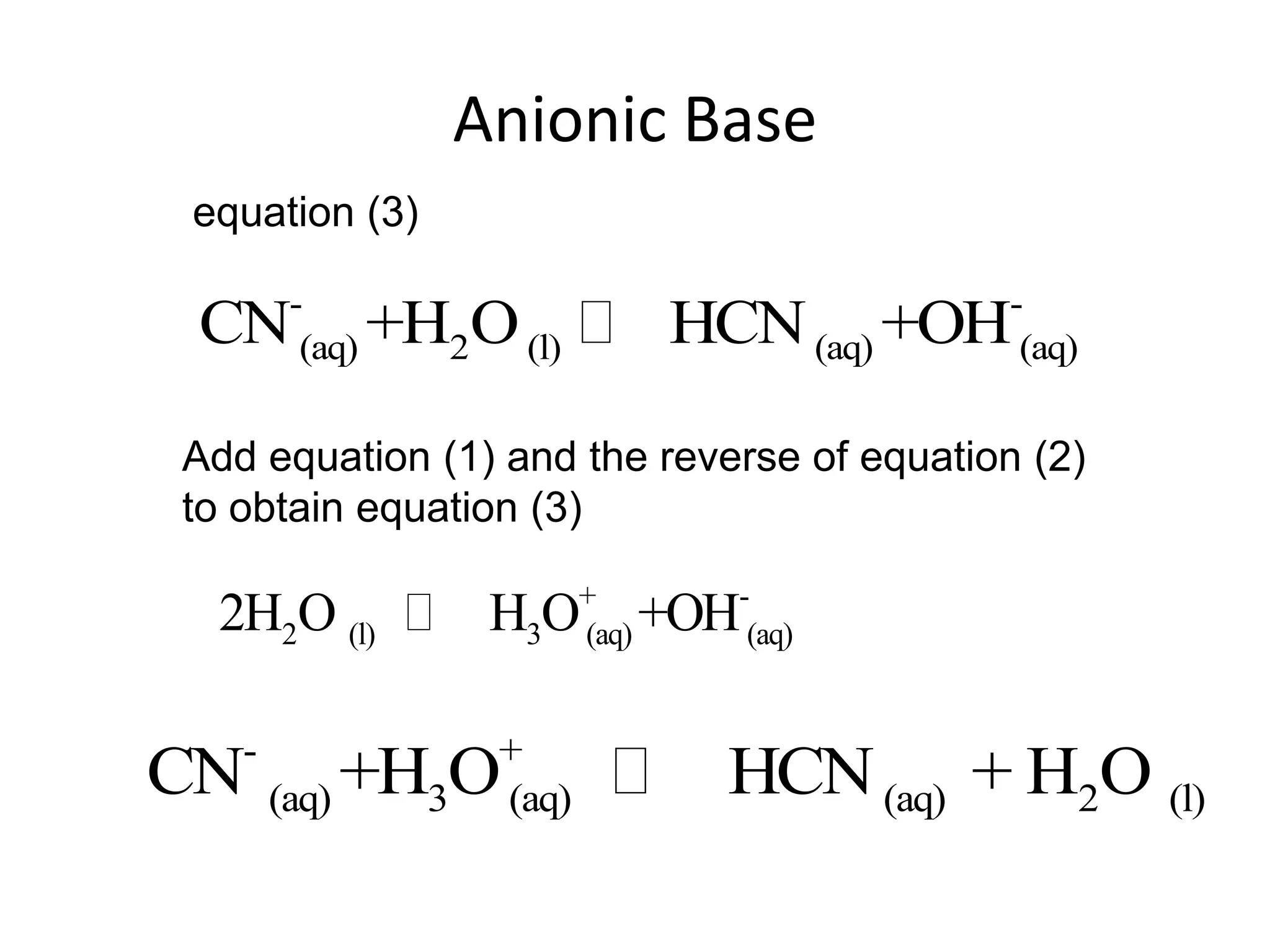
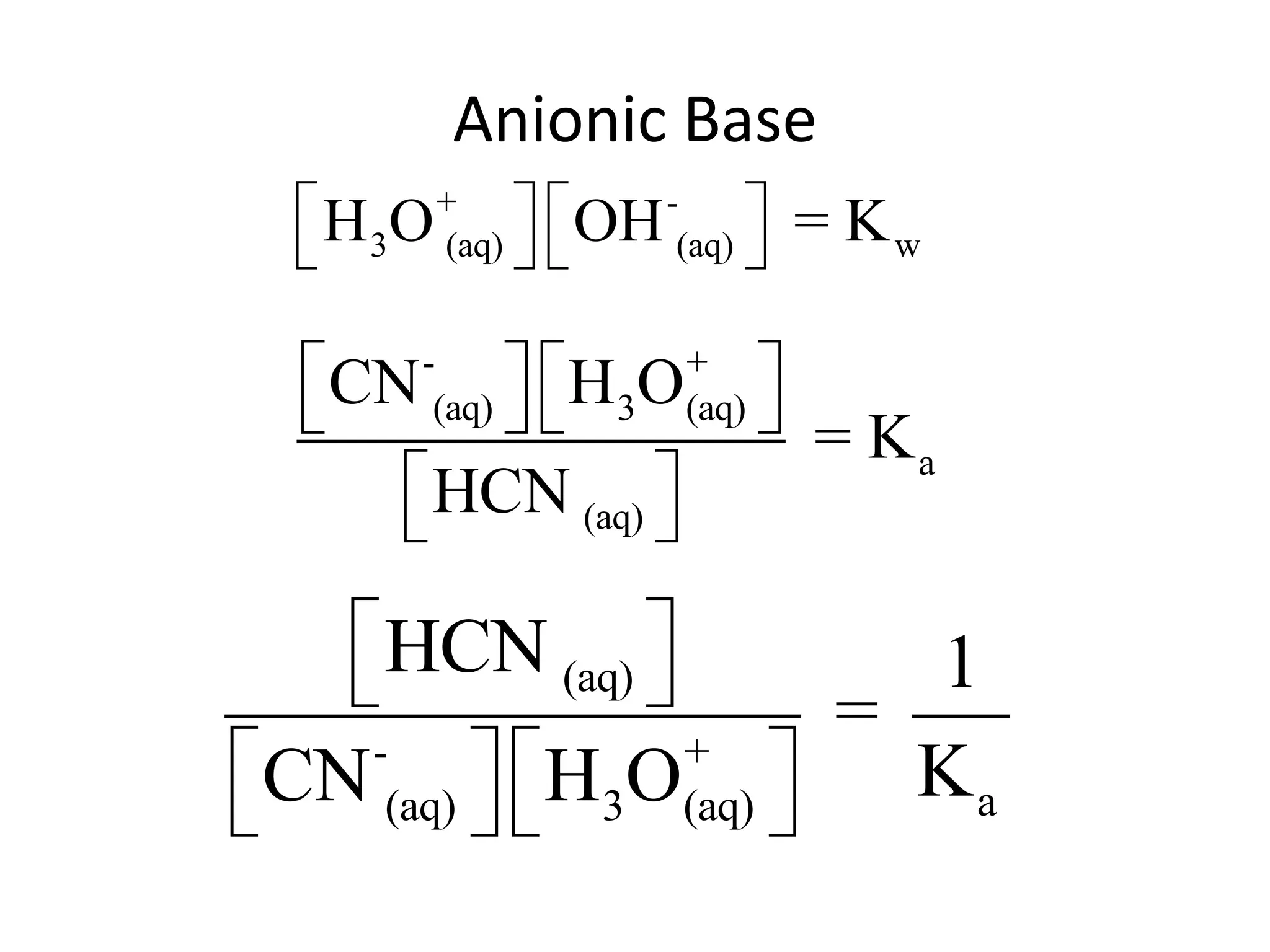
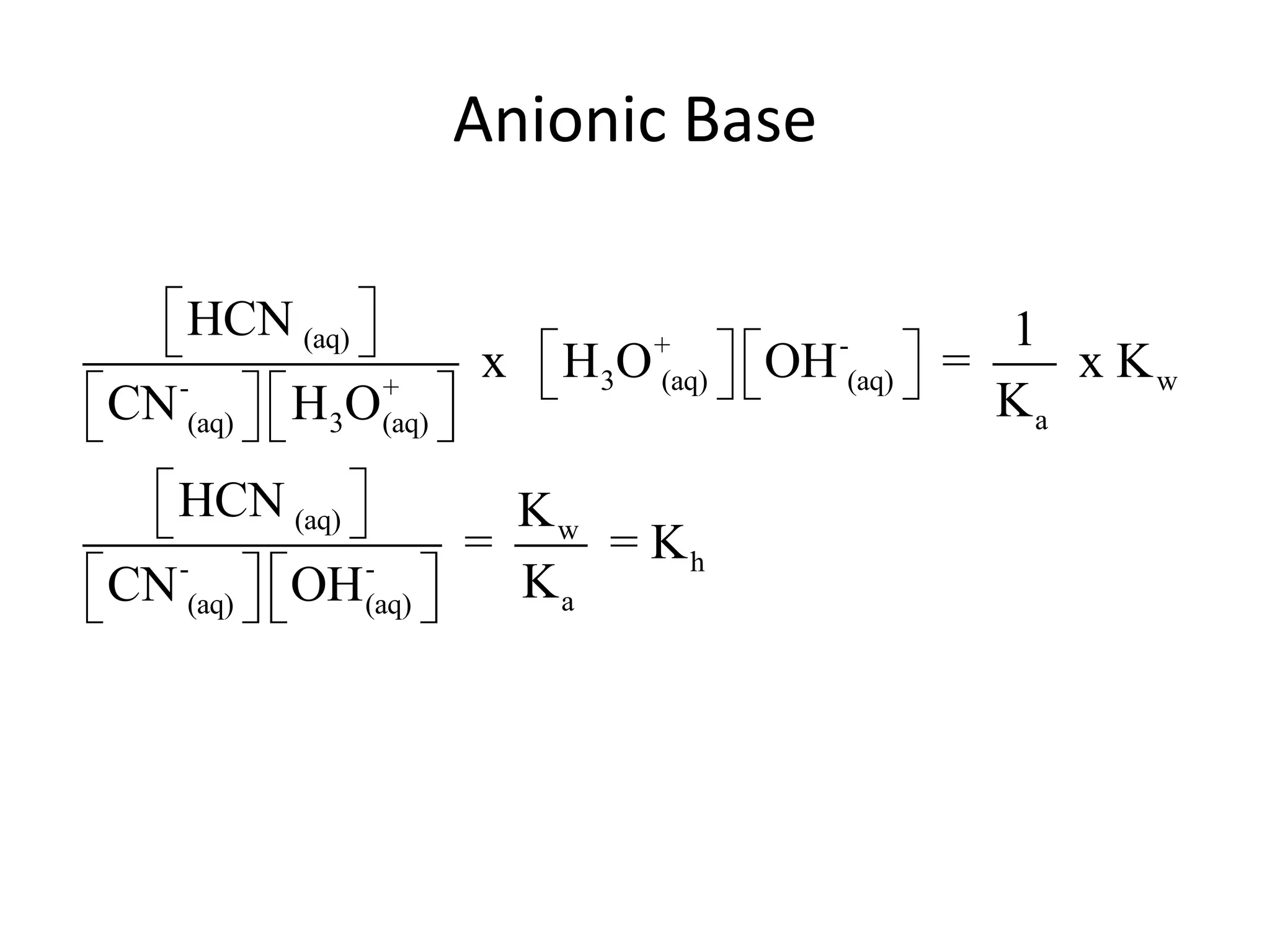
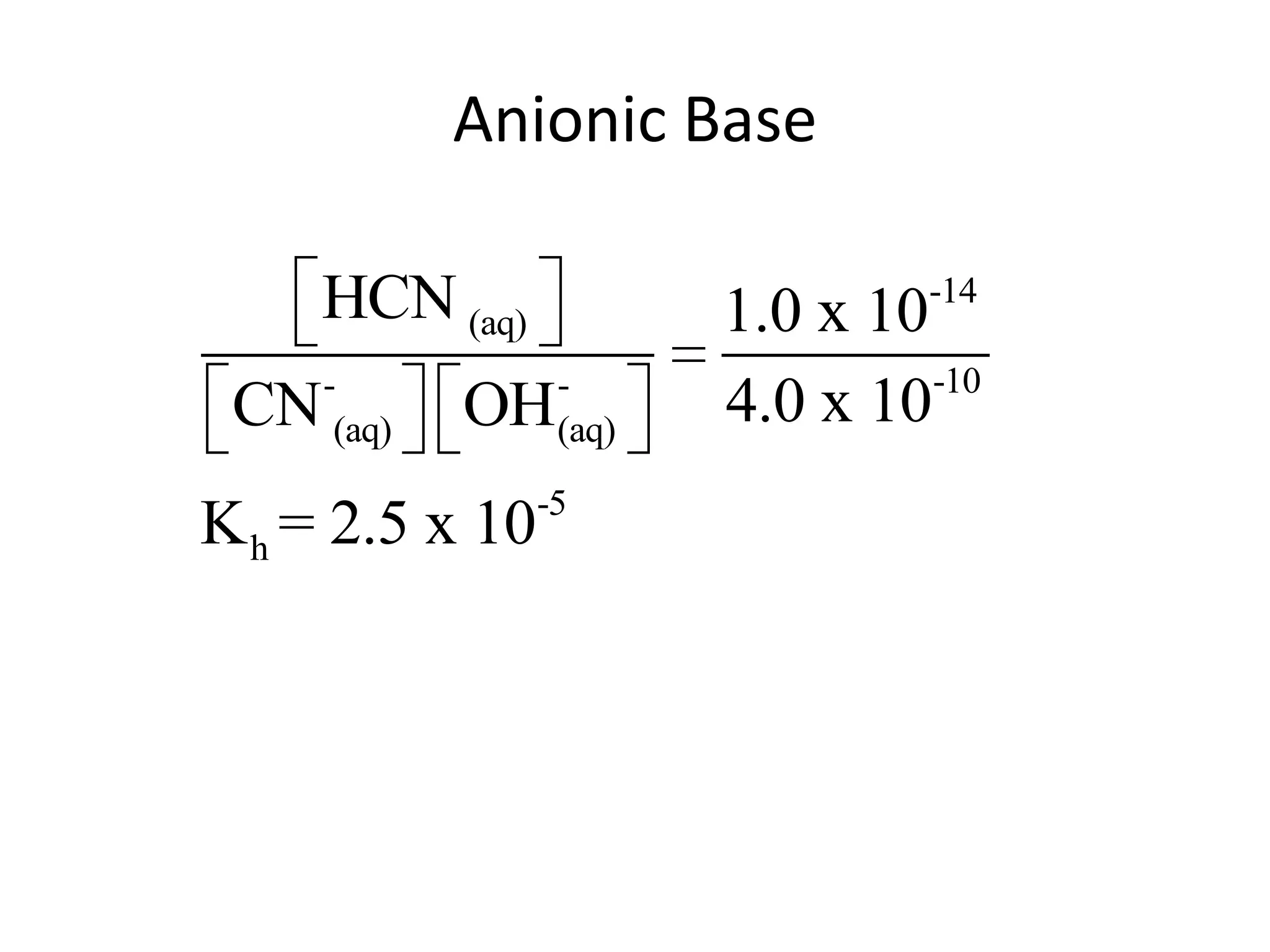
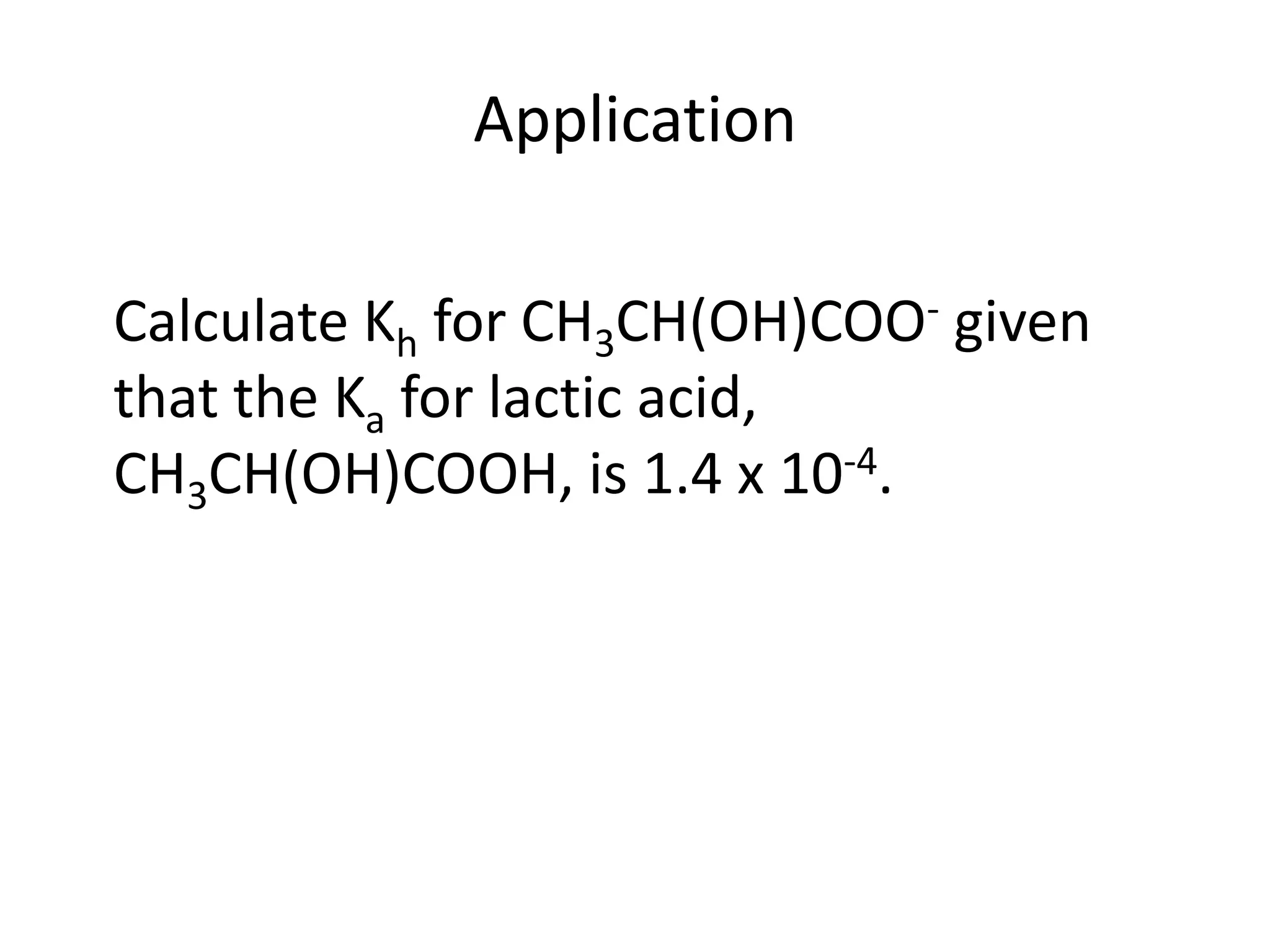
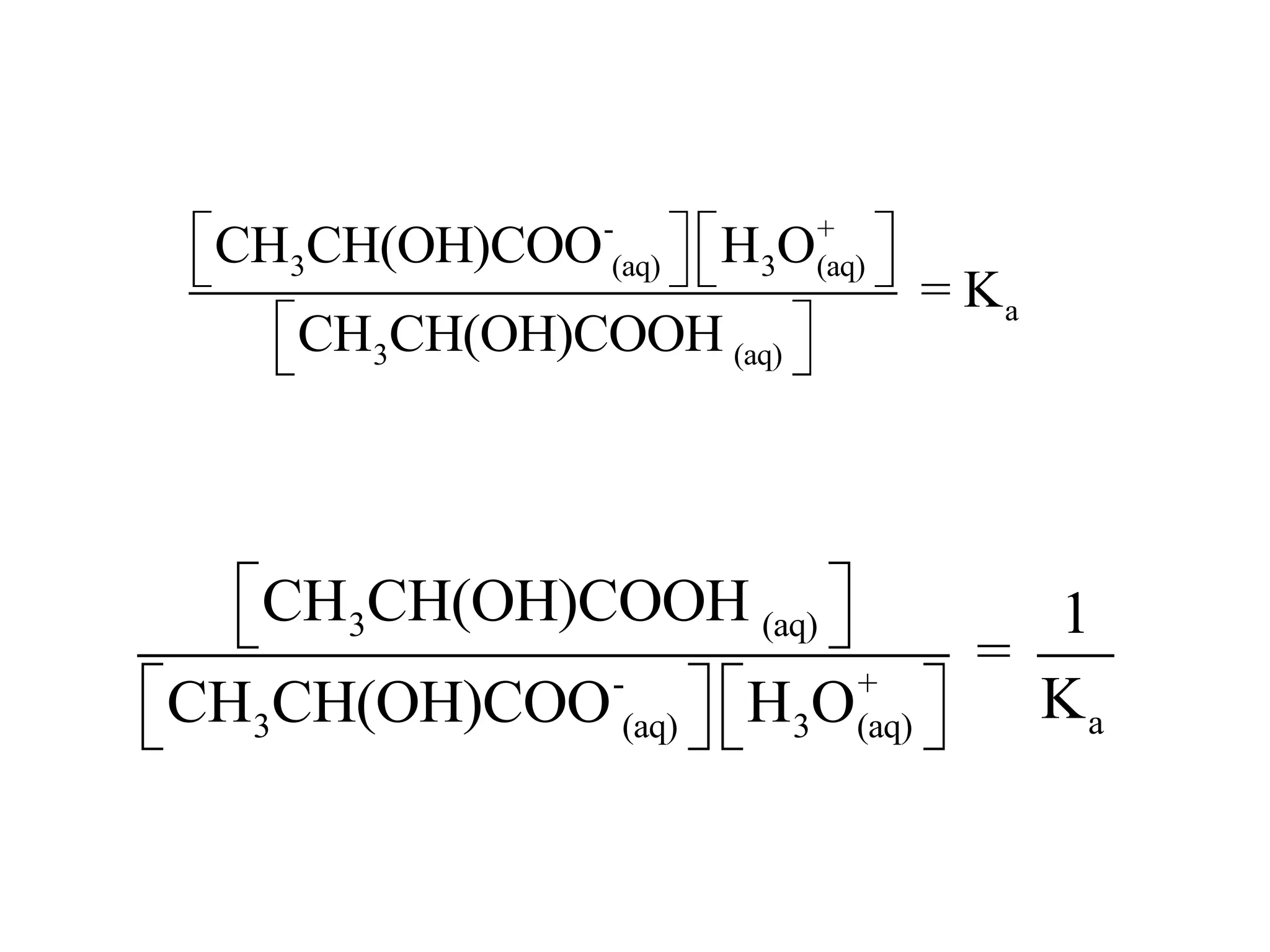
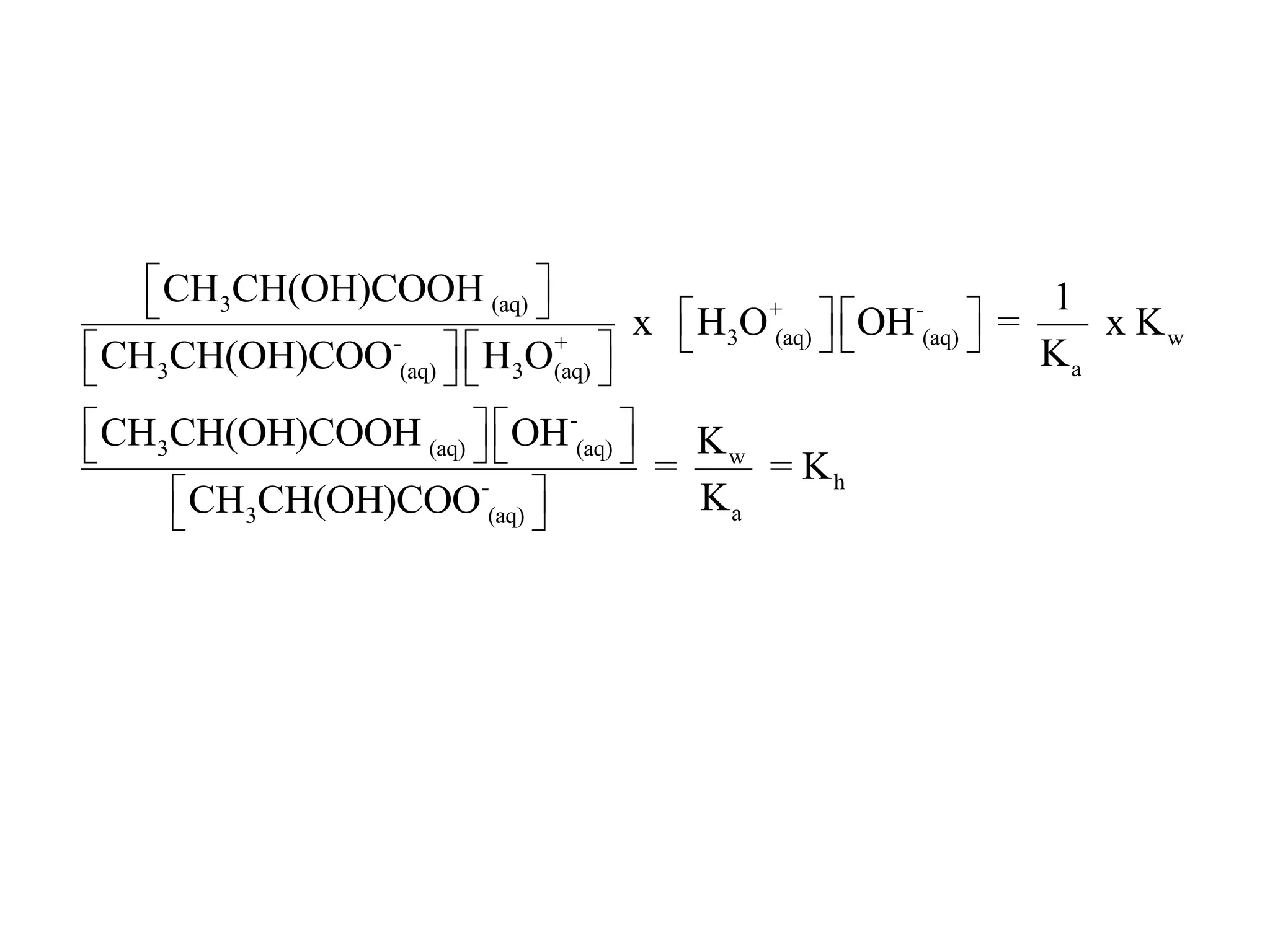
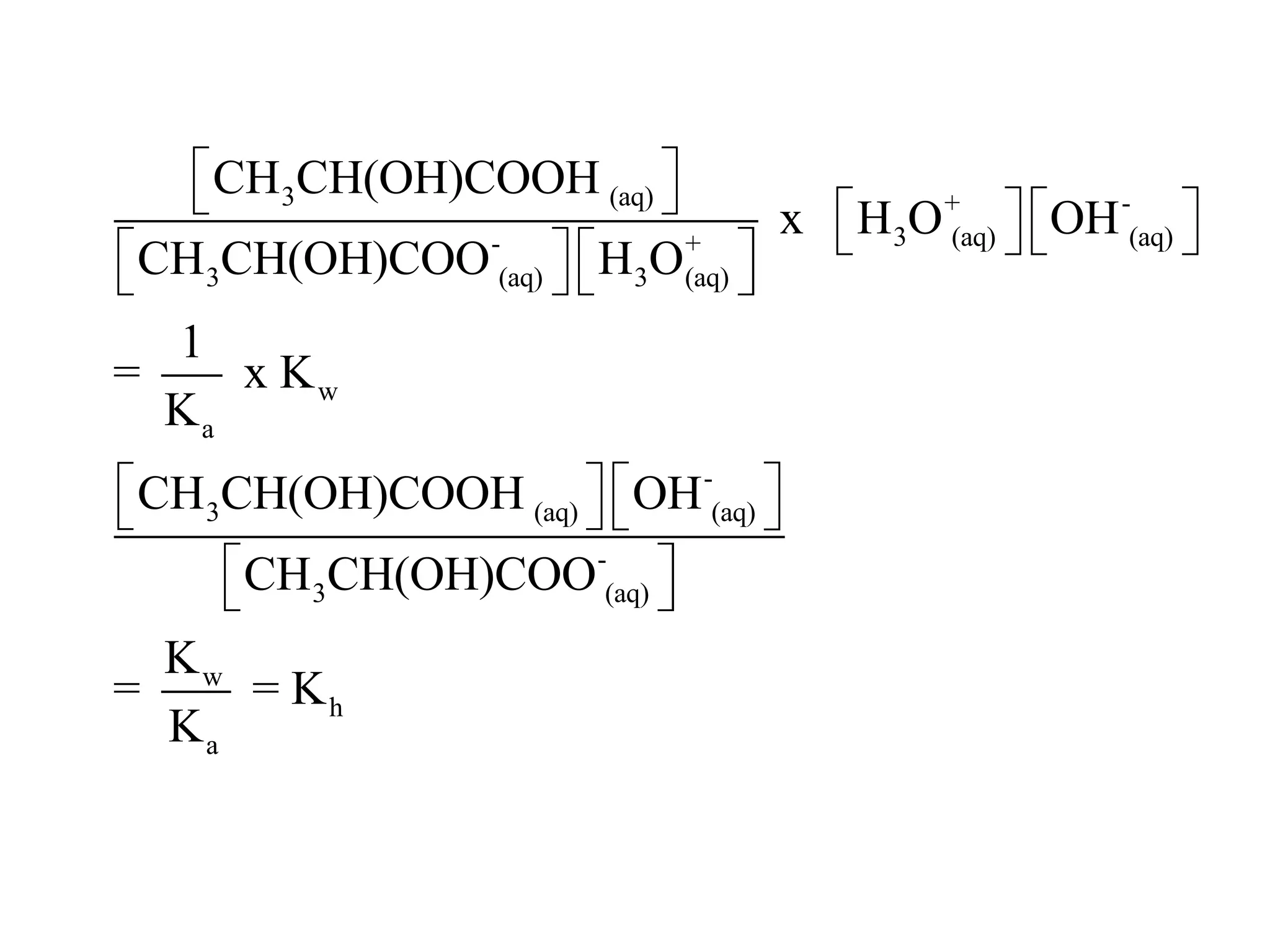
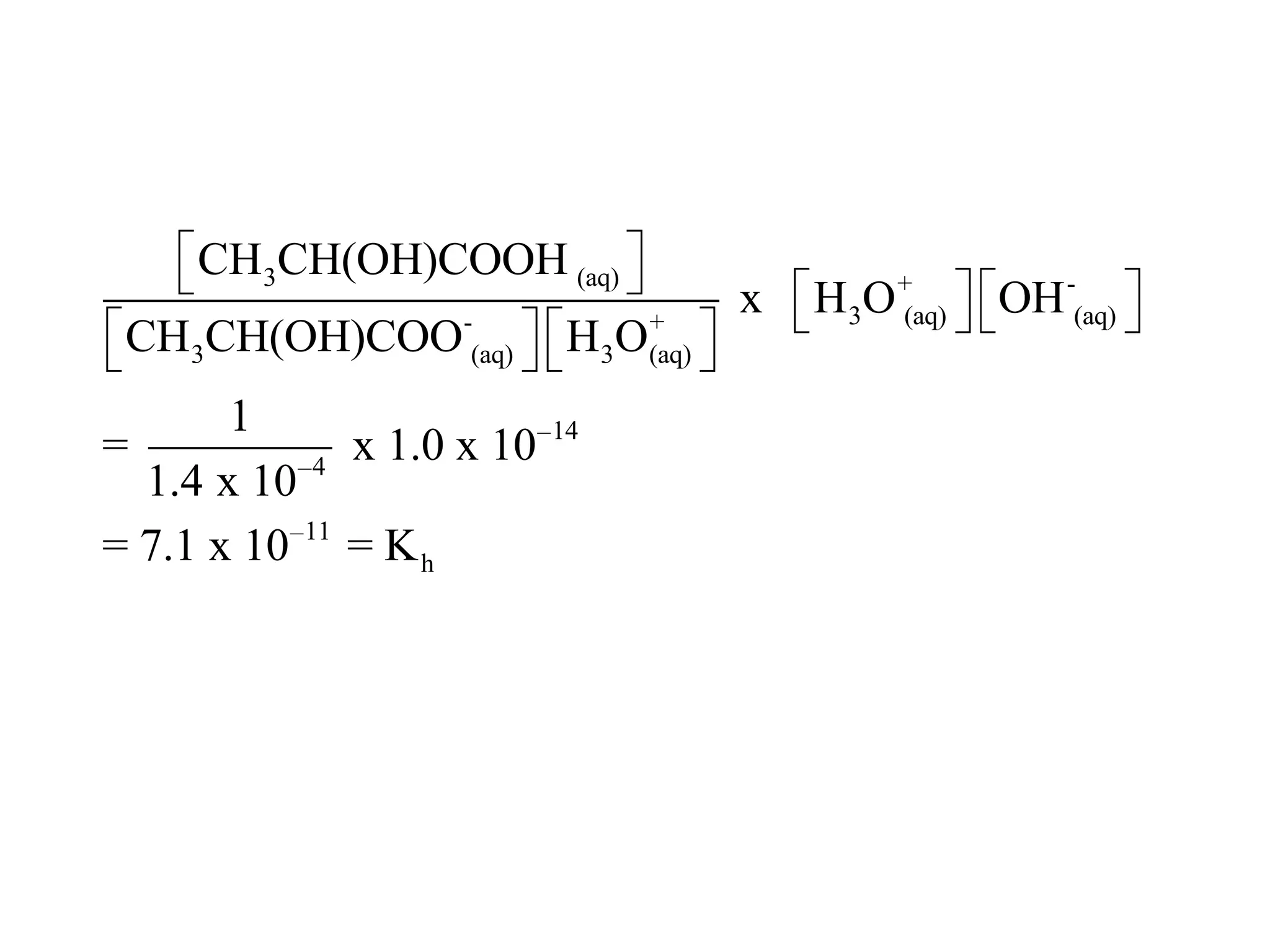
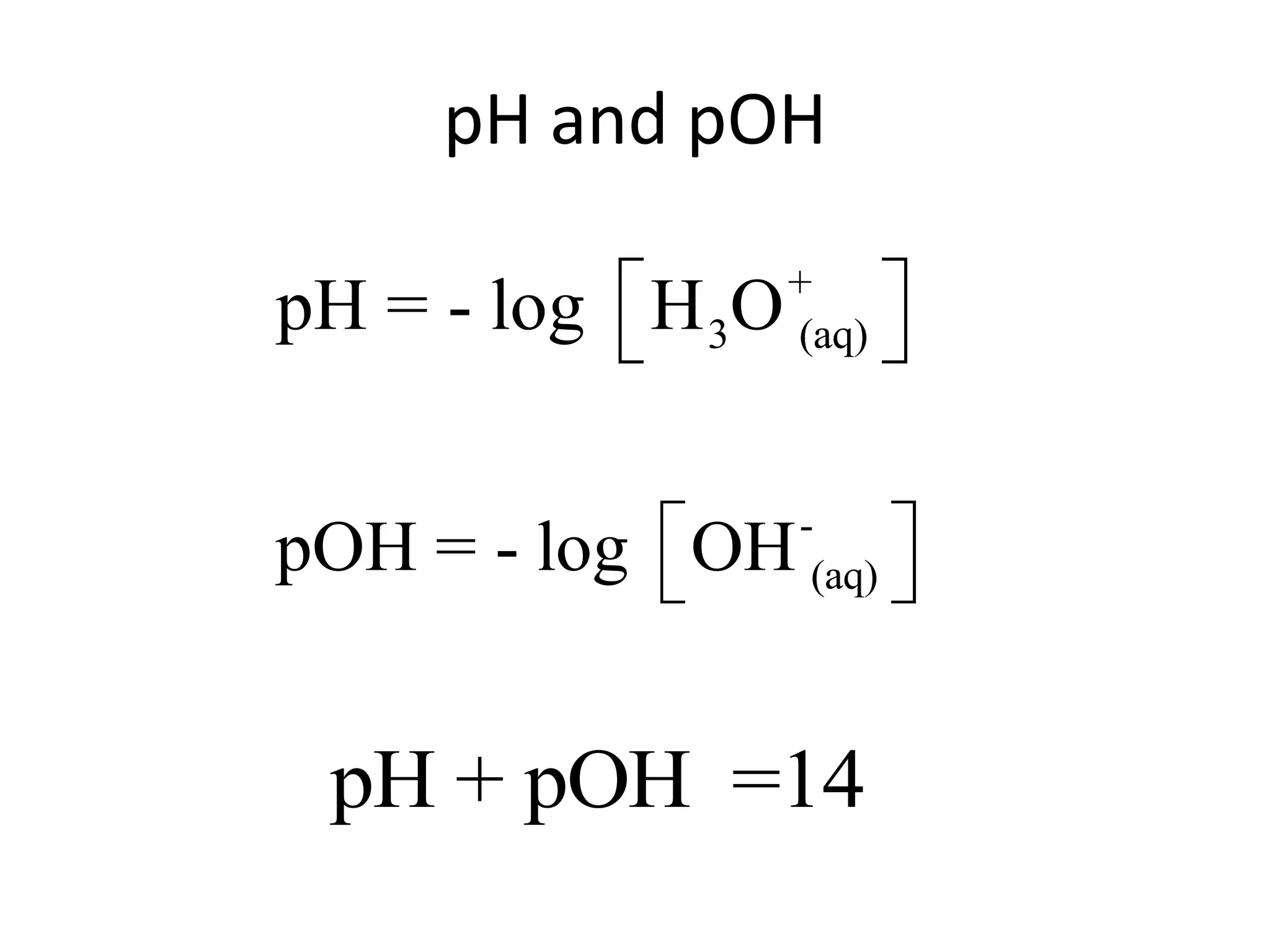
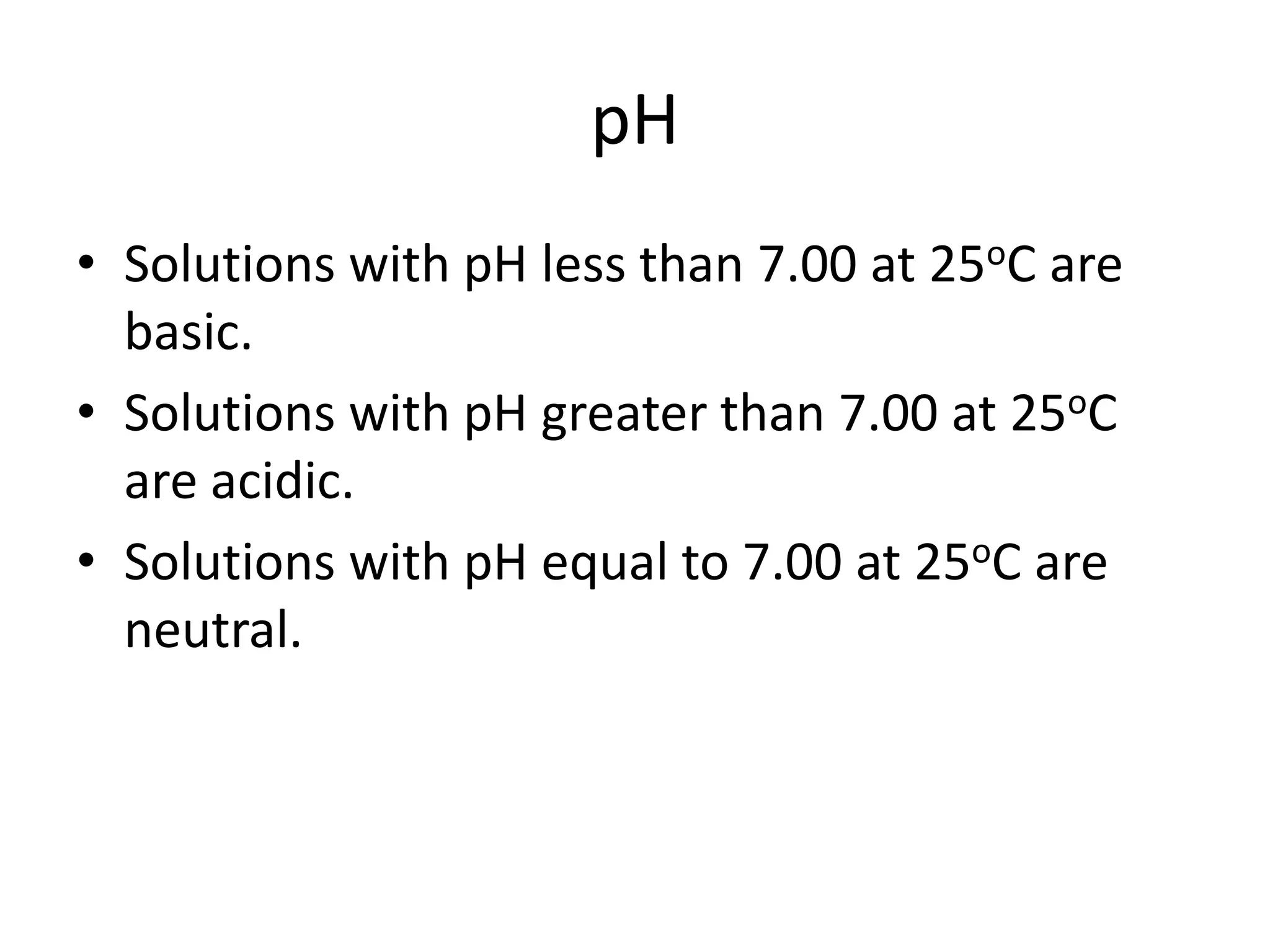

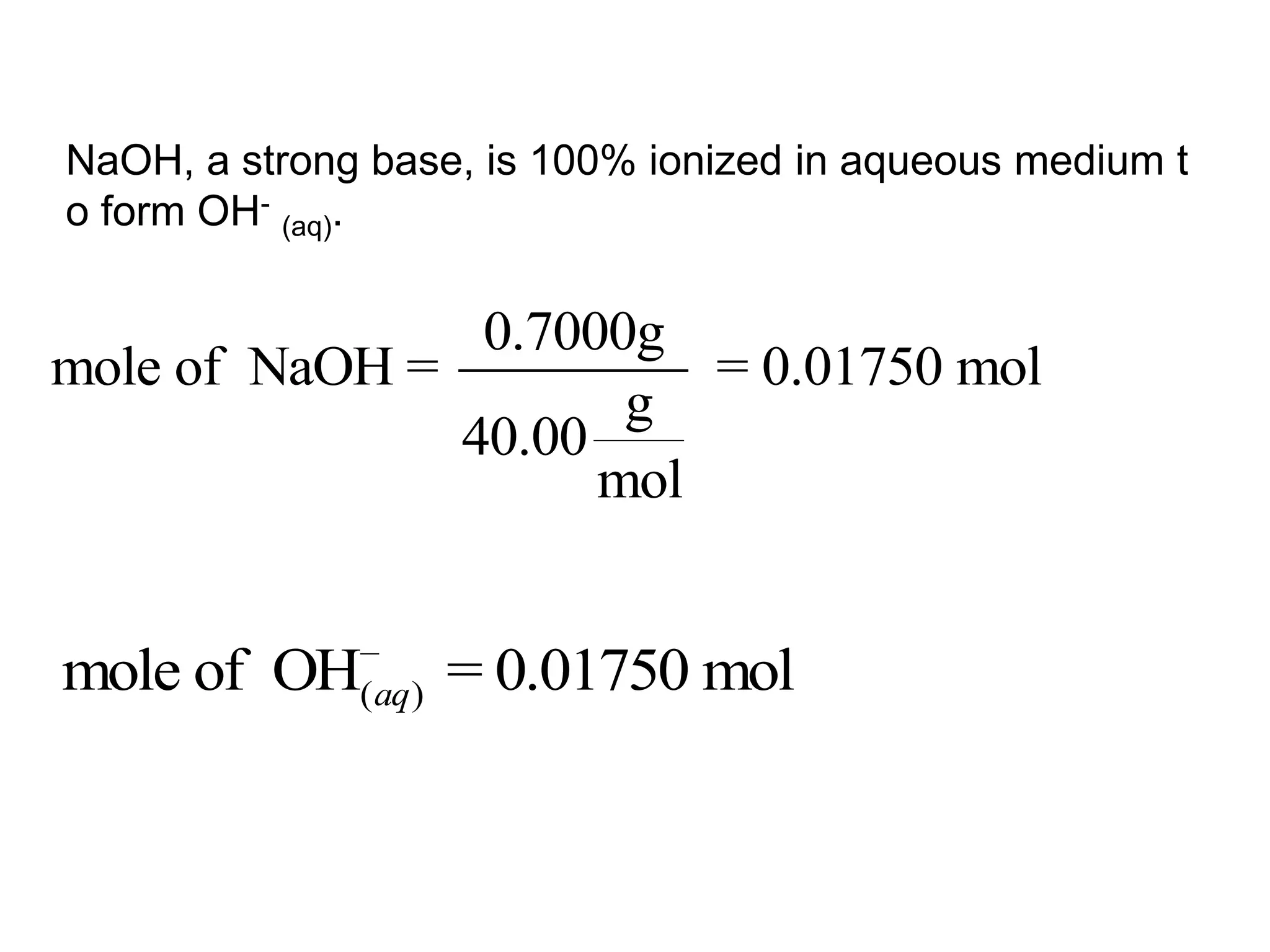
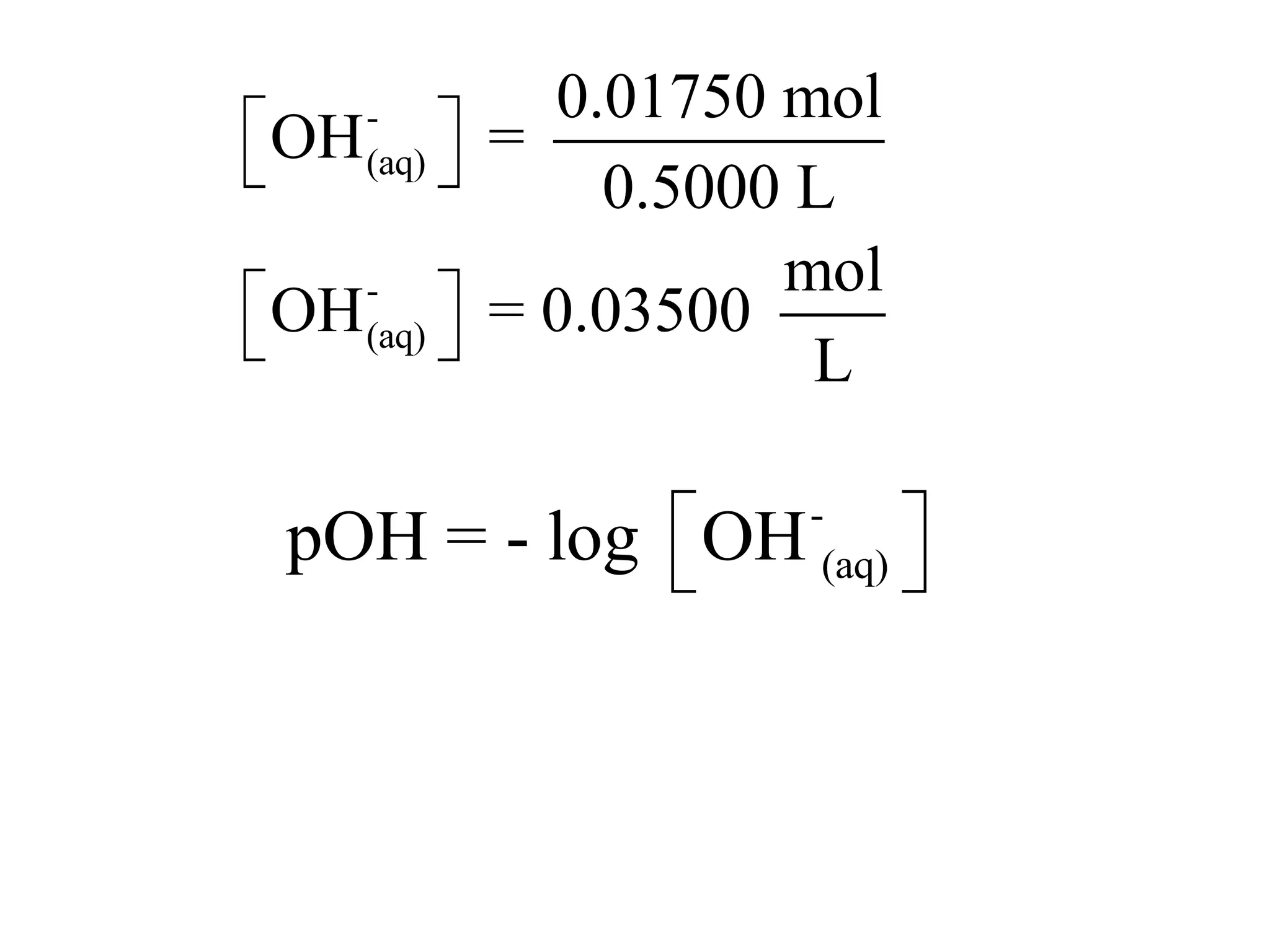

![ApplicationIf [H3O+] in vinegar is 1.6 x 10-3, calculate its pH.](https://image.slidesharecdn.com/acidbaseequilibrium-100610183655-phpapp01/75/Acid-Base-Equilibrium-36-2048.jpg)
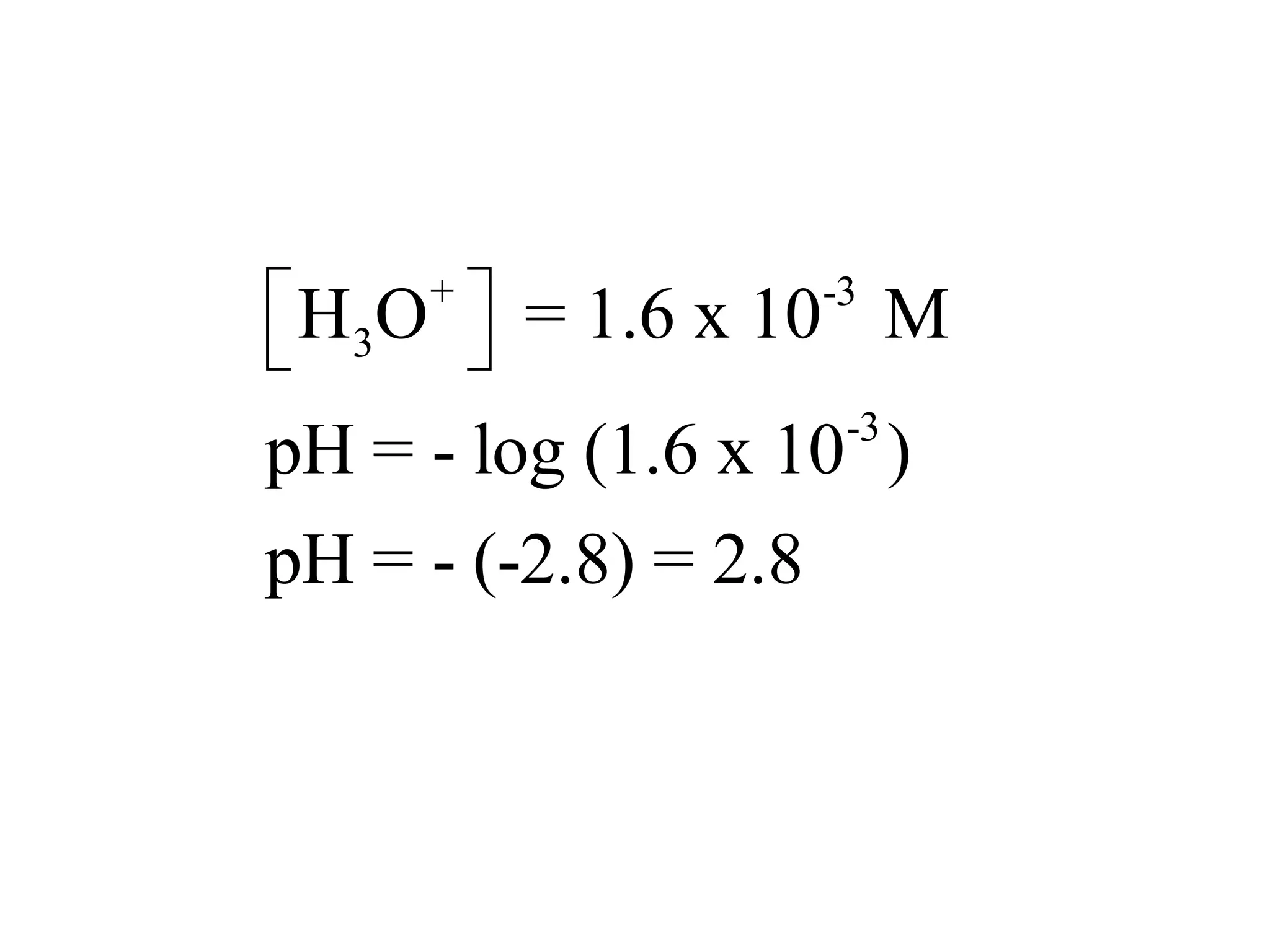
![Application The pH of seawater is 8.30. Calculate the [H3O+] and [-OH] of seawater.](https://image.slidesharecdn.com/acidbaseequilibrium-100610183655-phpapp01/75/Acid-Base-Equilibrium-38-2048.jpg)