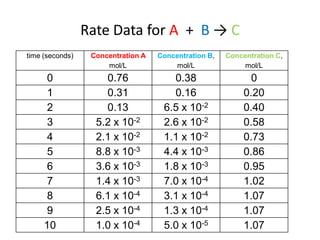
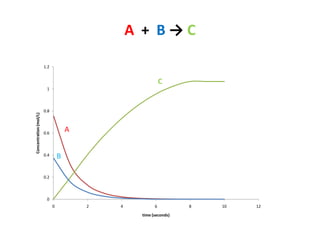
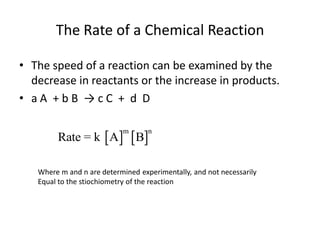
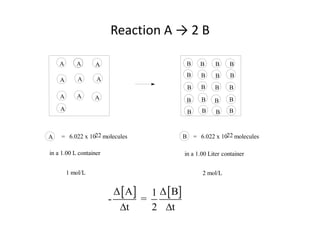
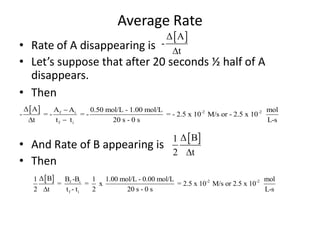
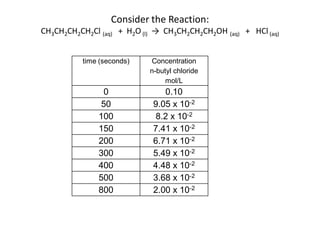
The document discusses chemical kinetics, which is the study of the speed of chemical reactions and the factors that affect reaction rates. It provides information on determining reaction rates from experimental data by measuring changes in reactant and product concentrations over time. The rate of a reaction is directly proportional to the concentrations of reactants raised to a power, where the powers are determined experimentally. Several examples are provided to illustrate how to calculate average and instantaneous reaction rates from concentration-time data and use this to determine the order of a reaction.

![mol
Average Rates, L-s
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
0.0 0.1000 1.90 x 10-4
50.0 0.0905
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
50.0 0.0905 1.70 x 10-4
100.0 0.0820](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-10-320.jpg)
![mol
Average Rates, L-s
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
100.0 0.0820 1.58 x 10-4
150.0 0.0741
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
150.0 0.0741 1.74 x 10-4
200.0 0.0671](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-11-320.jpg)
![mol
Average Rates, L-s
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
200.0 0.0671 1.22 x 10-4
300.0 0.0549
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
300.0 0.0549 1.01 x 10-4
400.0 0.0448](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-12-320.jpg)
![mol
Average Rates, L-s
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
400.0 0.0448 8.00 x 10-5
500.0 0.0368
time, seconds [CH3CH2CH2CH2Cl] Average Rate, mol
L-s
[C4 H9Cl]
t
500.0 0.0368 5.60 x 10-5
800.0 0.0200](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-13-320.jpg)
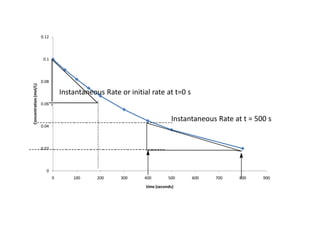

![IF Zero Order
time (seconds) [C4H9Cl]
0 0.10
50 9.05 x 10-2
100 8.2 x 10-2
150 7.41 x 10-2
200 6.71 x 10-2
300 5.49 x 10-2
400 4.48 x 10-2
500 3.68 x 10-2
800 2.00 x 10-2](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-18-320.jpg)
![60
50
40
[n-butyl chloride]
30
20
10
0
0 100 200 300 400 500 600 700 800 900
time (seconds)
Therefore, the reaction is not zero order](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-19-320.jpg)
![If Second Order
time (seconds) 1/[C4H9Cl]
0 10
50 11.0
100 12.2
150 13.5
200 14.9
300 18.2
400 22.3
500 27.2
800 50](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-20-320.jpg)
![60
50
40
1/[n-butyl chloride]
30
20
10
0
0 100 200 300 400 500 600 700 800 900
time (seconds)
Therefore, the reaction is not second order](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-21-320.jpg)
![IF First Order Reaction
time log [C4H9Cl] ln[C4H9Cl]
(seconds)
0 -1 -2.3
50 -1.04 -2.4
100 -1.09 -2.51
150 -1.13 -2.60
200 -1.17 -2.69
300 -1.26 -2.90
400 -1.35 -3.11
500 -1.43 -3.29
800 -1.7 -3.92](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-22-320.jpg)
![First Order Plot
0
0 100 200 300 400 500 600 700 800 900
-0.2
-0.4
-0.6
log [n-butyl chloride]
-0.8
-1
-1.2 y = -0.0009x - 0.9985
-1.4
-1.6
-1.8
time (seconds)](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-23-320.jpg)
![First Order Plot
0
0 100 200 300 400 500 600 700 800 900
-0.5
-1
-1.5
ln[C4H9Cl]
-2
-2.5
-3 y = -0.002x - 2.2987
-3.5
-4
-4.5
time (seconds)](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-24-320.jpg)
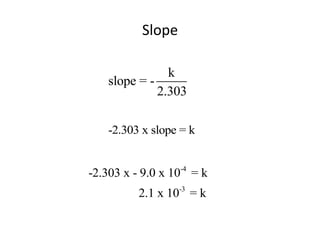
![Rate of the Reaction
Rate = k [n-butylchloride]](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-27-320.jpg)
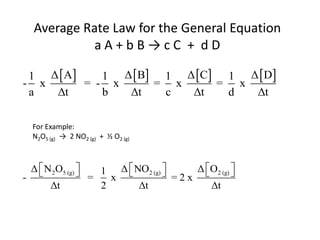
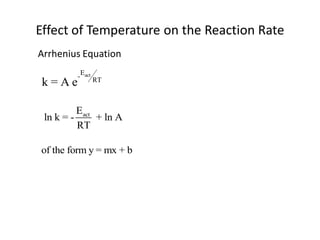
![For the Reaction
N2O5 (g) → 2 NO2 (g) + ½ O2 (g)
Rate = k [N2O5 ]
The rate can be used to explain the mechanism](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-28-320.jpg)
![Application
Mechanism of a Chemical Reaction
(a)
Suggest a possible mechanism for
NO2 (g) + CO (g) → NO (g) + CO2 (g)
Given that
Rate = k [NO2(g) ]2
(b)
Suggest a possible mechanism for
2 NO2 (g) + F2 (g) → 2 NO2F (g)
Given that
Rate = k [NO2 (g) ] [F2 (g) ]](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-31-320.jpg)

![A Second Order Reaction
H2O2 (aq) + I (aq) H2O (l) + O2 (g)
-
-
Rate = k [H2O2(aq) ] [I(aq) ]](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-34-320.jpg)

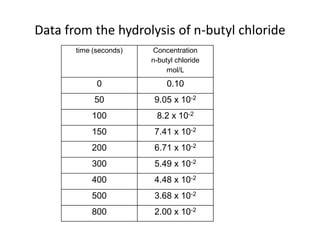
![The following data were collected for the rate of the reaction
Between A and B, A + B → C , at 25oC. Determine the rate law
for the reaction and calculate k.
Experiment [A], moles/L [B], moles/L Initial Rate, M/s
1 0.1000 0.1000 5.500 x 10-6
2 0.2000 0.1000 2.200 x 10-5
3 0.4000 0.1000 8.800 x 10-5
4 0.1000 0.3000 1.650 x 10-5
5 0.1000 0.6000 3.300 x 10-5](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-36-320.jpg)
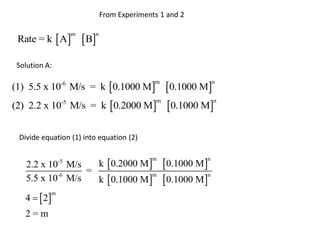
![Solution B:
(1) log (5.5 x 10-6 ) = log k + m log 0.1000 + n log 0.1000
(2) log (2.2 x 10-5 ) = log k + m log 0.2000 + n log 0.1000
Subtract equation (2) from equation (1)
log (5.5 x 10-6 ) -log (2.2 x 10-5 ) = m [log 0.1000 -log 0.2000]
-5.3 - (-4.7) = m [-1 - (-0.7)]
-0.6 = m [-0.3]
-0.6
=m
-0.3
2=m](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-38-320.jpg)
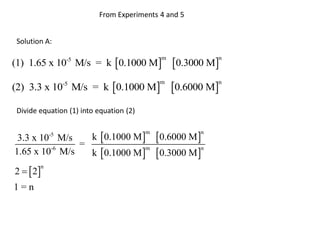
![Solution B:
(1) log (1.65 x 10-5 ) = log k + m log 0.1000 + n log 0.3000
(2) log (3.3 x 10-5 ) = log k + m log 0.1000 + n log 0.6000
Subtract equation (2) from equation (1)
log (1.65 x 10-5 ) -log (3.3 x 10-5 ) = n [log 0.3000 -log 0.6000]
-4.78 - (-4.5) = n [-0.5227 - (-0.2218)]
-0.3 = n [-0.3]
-0.3
=n
-0.3
1=n](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-40-320.jpg)
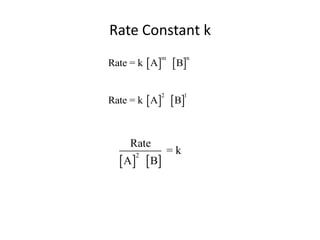
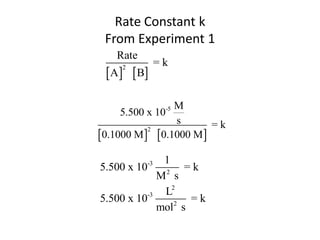
![Your Understanding of this Process
Consider the Data for the Following Reaction:
O O
_
CH3 C + OH CH3 C + CH3OH
_
OCH3 O
Experiment [CH3CO2CH3] [-OH] Initial Rate,
M M M/s
1 0.050 0.050 0.00034
2 0.050 0.100 0.00069
3 0.100 0.100 0.00137
Determine the Rate Law Expression and the value of k consistent
With these data.](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-44-320.jpg)
![From Experiments 1 and 2
n
Rate = k [CH3CO2CH3 ] m
- OH
Solution :
(1) 3.4 x 10 M/s = k 0.050 M 0.050 M
-4 m n
(2) 6.9 x 10-4 M/s = k 0.50 M 0.100 M
m n
Divide equation (1) into equation (2)
k 0.050 M 0.100 M
-4 m n
6.9 x 10 M/s
=
k 0.050 M 0.050 M
-5 m n
3.4 x 10 M/s
2 2
n
1=n](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-45-320.jpg)
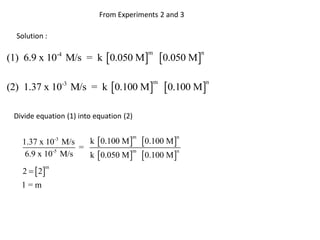
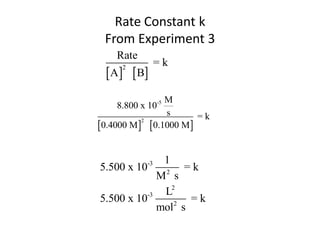
![Rate Expression
Rate = k [CH3CO2CH3 ] [ - OH]
Rate
-
=k
[CH3CO2CH3 ] [ OH]
M
0.00137
s =k
[0.100 M] [0.100 M]
1
0.137 =k
Ms
L
0.137 =k
mol s](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-47-320.jpg)
![Assignment
Determine the Rate Law for the following reaction from the
given data:
2 NO (g) + O2 (g) → 2 NO2 (g)
Experiment [NO (g)] [O2 (g)] Initial Rate,
M M M/s
1 0.020 0.010 0.028
2 0.020 0.020 0.057
3 0.020 0.040 0.114
4 0.040 0.020 0.227
5 0.010 0.020 0.014](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-48-320.jpg)
![Data for the Transformation of cylcpropane to propene
ao X ao –x Ln[ao]/[ ao –x] t
M M M seconds
0.050 0 0.050 0 0
0.050 0.0004 0.0496 9.0 x 10-3 600
0.050 0.0009 0.0491 0.0180 1200
0.050 0.0015 0.0485 0.0300 2000
0.050 0.0022 0.0478 0.045 3000
0.050 0.0036 0.0464 0.075 5000
0.050 0.0057 0.0443 0.120 8000
0.050 0.0070 0.0430 0.150 10000
0.050 0.0082 0.0418 0.180 12000](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-51-320.jpg)
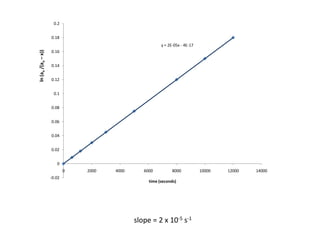
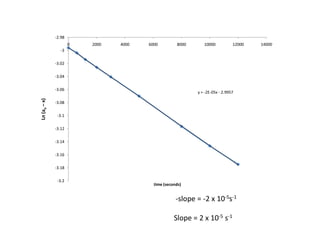
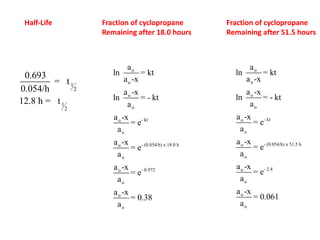
![Data for the Transformation of cylcpropane to propene
ao X ao –x Ln [ ao –x] t
M M M seconds
0.050 0 0.050 -2.996 0
0.050 0.0004 0.0496 -3.0038 600
0.050 0.0009 0.0491 -3.014 1200
0.050 0.0015 0.0485 -3.026 2000
0.050 0.0022 0.0478 -3.0407 3000
0.050 0.0036 0.0464 -3.070 5000
0.050 0.0057 0.0443 -3.1167 8000
0.050 0.0070 0.0430 -3.1466 10000
0.050 0.0082 0.0418 -3.1748 12000](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-53-320.jpg)
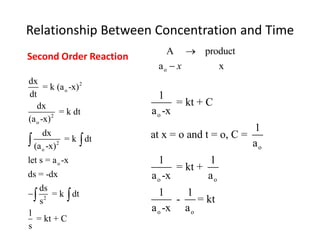
![Data for the Transformation of hydrogen iodide gas to hydrogen and iodine
ao X ao –x 1/[ ao –x] t
M M M M-1 Minutes
0.0100 0 0.0100 100 0.00
0.0100 0.0060 0.00400 250 5.00
0.0100 0.0075 0.00250 400 10.0
0.0100 0.0086 0.00143 700 20.0
0.0100 0.0090 0.0010 1000 30.0
0.0100 0.0099 0.00077 1300 40.0
0.0100 0.0094 0.00063 1600 50.0
0.0100 0.0095 0.00053 1900 60.0](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-57-320.jpg)
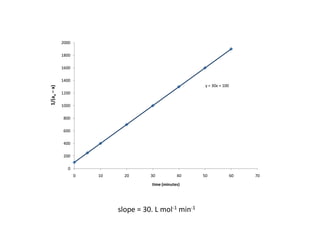
![Application of the Graphical Method for Determining
the Order of a Reaction
N2O5 (g) → 2 NO2 (g) + ½ O2 (g)
[ N 2O 5 ] t
M minutes
2.08 3.07
1.67 8.77
1.36 14.45
0.72 31.28
Tabulate the data so that each order may be tested](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-61-320.jpg)
![Data tabulation to determine which order will give a linear graph
[ N2O5 ] t ln[ N2O5 ] 1/[ N2O5 ]
M minutes (first order) M-1
(zero order) (second order)
2.08 3.07 0.732 0.481
1.67 8.77 0.513 0.599
1.36 14.45 0.307 0.735
0.72 31.28 -0.329 1.390](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-62-320.jpg)
![Test for zero order reaction
2.5
2
1.5
[N2O5]
1
0.5
0
0 5 10 15 20 25 30 35
time (minutes)
Not linear; therefore, the reaction is not zero order](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-63-320.jpg)
![Test for first order reaction
0.8
0.6
y = -0.0376x + 0.8462
0.4
ln[N2O5]
0.2
0
0 5 10 15 20 25 30 35
-0.2
-0.4
time (minutes)
Linear; therefore, the reaction is first order](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-64-320.jpg)
![Test for second order reaction
1.6
1.4
1.2
1
1/[N2O5]
0.8
0.6
0.4
0.2
0
0 5 10 15 20 25 30 35
time (minutes)
Non-linear; therefore, the reaction is not second order](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-65-320.jpg)
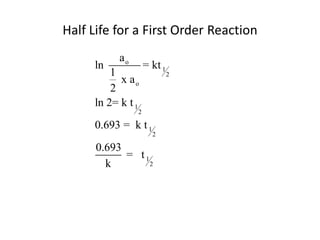
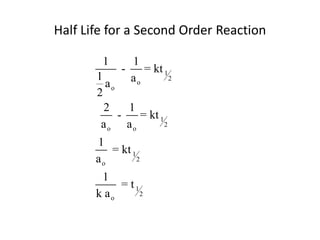
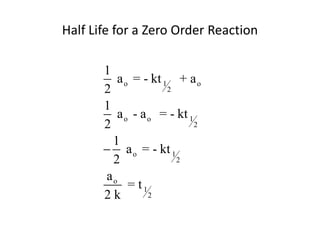

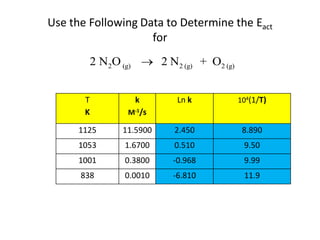
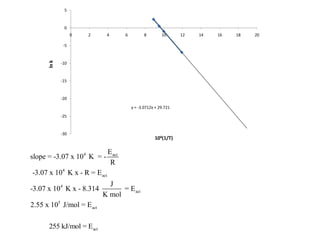
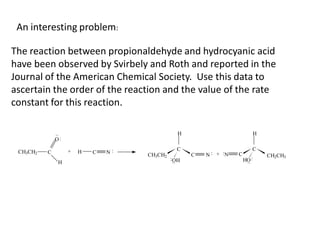
![time, minutes [HCN] [CH3CH2CHO]
2.78 0.0990 0.0566
5.33 0.0906 0.0482
8.17 0.0830 0.0406
15.23 0.0706 0.0282
19.80 0.0653 0.0229
0.0424 0.0000
∞](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-79-320.jpg)
![Check to determine first order in HCN
-2.35
0 2 4 6 8 10 12 14 16 18
-2.4
-2.45
-2.5
ln([HCN]-x)
-2.55
-2.6
-2.65
-2.7
-2.75
time (minutes)](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-80-320.jpg)
![Check to determine first order in propionaldehyde
0
0 2 4 6 8 10 12 14 16 18
-0.5
time (minutes)
-1
ln ([propionaldehyde] -x)
-1.5
-2
-2.5
-3
-3.5
-4](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-81-320.jpg)
![So close; therefore, let’s take another approach. Let [HCN] = ao and
[propionaldehyde] = bo
Then,
dx
= k (a o -x) (b o -x)
dt
dx
= k dt
(a o -x) (b o -x)
dx
(a o -x) (bo -x) = k dt
Solution:
1 (a o -x) 1 bo
ln = kt - ln
(a o -bo ) b o -x (a o -b o ) a o](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-82-320.jpg)
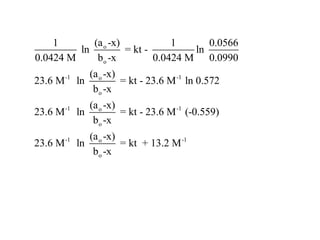
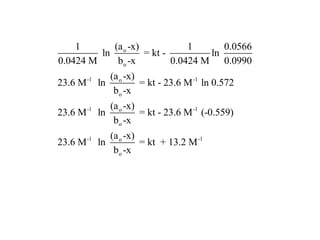
![Let’s construct the data in a different format
time, minutes [HCN] - x [CH3CH2CHO]-x ([HCN]-x)
(23.6) ln
([CH3CH2 CHO]-x)
2.55 0.0906 0.0482 14.9
5.39 0.0830 0.0406 16.9
12.76 0.0706 0.0282 21.7
17.02 0.0653 0.0229 24.8](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-85-320.jpg)
![30
25
20
([HCN] - x) y = 0.6778x + 13.184
23.6 ln
([CH3CH 2CHO]-x)
15
10
5
0
0 2 4 6 8 10 12 14 16 18
time, minutes
Slope = 0.678; therefore, k = 0.678 M-1min-1](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-86-320.jpg)
![Rate = k [HCN] [CH3CH2CHO]
Mechanism:
k1
+ -
1. HCN + H2O H3O + CN
k -1
+
O OH
k2
2. + + H2O
CH3CH2 C + H3O CH3CH2 C
k-2
H H
+ H
3. OH
k3
- C
CH3CH2 C + CN
CH3CH2 CN
H HO](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-87-320.jpg)
![Steps 1 and 2 are fast equilibrium steps; and step 3 is the rate determining step
+
OH
Rate = k3 CH3CH2 [ CN- ]
C
H
k 1 [HCN] [H2O] = k -1 [H3O+] [CN-]
k 1 [HCN] [H2O]
= [CN-]
k -1 [H3O+]](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-88-320.jpg)
![O
CH3CH2 C +
[H3O ]
k +
2
H OH
= CH3CH2 C
k [H2O] H
-2
O
CH3CH2 C
+
k3 k1 k2 [HCN] [H2O] H [H3O ]
Rate =
+
k -1 k [H3O ] [H2O]
-2](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-89-320.jpg)
![O
Rate = k [HCN] CH3CH2 C
H](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-90-320.jpg)
![Revisit the kinetics for
2 NO (g) + O2 (g) → 2 NO2 (g)
Rate = k [NO]2 [O2 ]](https://image.slidesharecdn.com/gcchemicalkinetics-110518143920-phpapp02/85/GC-Chemical-Kinetics-91-320.jpg)