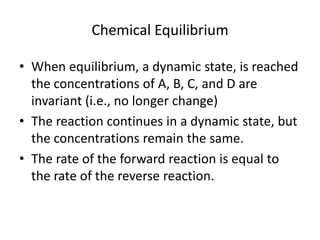
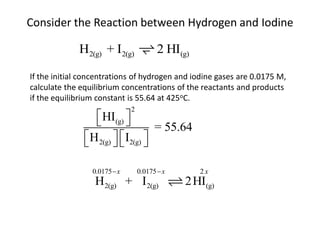
The document discusses chemical equilibrium for the reaction aA + bB ⇌ cC + dD. It provides the rate expressions for the forward and reverse reactions and shows that at equilibrium, these rates are equal. Graphs illustrate how the concentrations of reactants and products remain constant once equilibrium is reached, while the rates continue at the same value. The document also discusses how to calculate equilibrium constants and manipulate equilibrium expressions.
![Chemical Equilibrium
k1
aA+bB cC+dD
k-1
rate of the forward reaction = k1 [A]a [B]b
rate of the reverse reaction = k -1 [C]c [D]d
At equilibrium:
rate of the forward reaction = rate of the reverse reaction
k1 [A]a [B]b = k -1 [C]c [D]d](https://image.slidesharecdn.com/gcchemicalequilibrium-110526013040-phpapp02/85/Gc-chemical-equilibrium-1-320.jpg)
![Chemical Equilibrium
k1 [A]a [B]b k -1 [C]c [D]d
k1 [C]c [D]d
a b
k -1 [A] [B]
[C]c [D]d
K eq
[A]a [B]b](https://image.slidesharecdn.com/gcchemicalequilibrium-110526013040-phpapp02/85/Gc-chemical-equilibrium-2-320.jpg)
![Chemical Equilibrium
Concentration versus time
1.2
at equilibrium
1
0.8 [C] and [D]
concentration, M
0.6
[A] and [B]
0.4
0.2
0
0 2 4 6 8 10 12 14 16 18 20
time (minutes)](https://image.slidesharecdn.com/gcchemicalequilibrium-110526013040-phpapp02/85/Gc-chemical-equilibrium-4-320.jpg)
![Chemical Equilibrium
Rate versus time
1.4 at equilibrium
1.2
k1 [A]a [B]b
1
k1 [A]a [B]b = k -1 [C]c [D]d
0.8
Rate
0.6
0.4
k -1 [C]c [D]d
0.2
0
0 2 4 6 8 10
time (minutes) 12 14 16 18](https://image.slidesharecdn.com/gcchemicalequilibrium-110526013040-phpapp02/85/Gc-chemical-equilibrium-5-320.jpg)


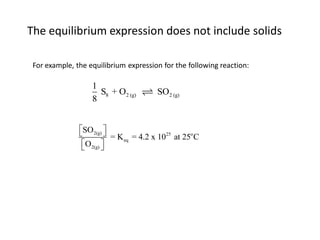
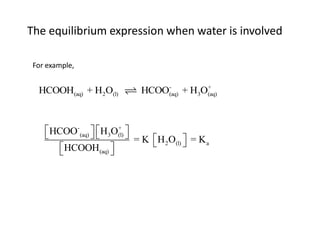
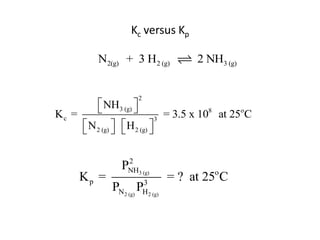
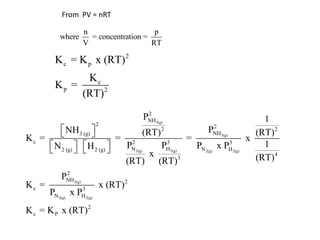
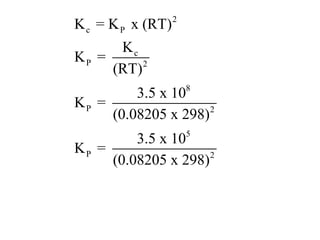
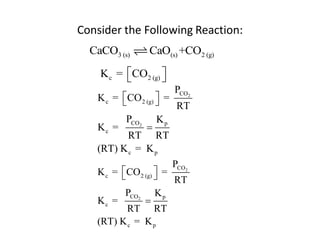
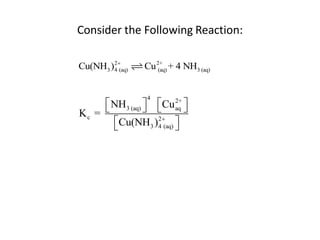
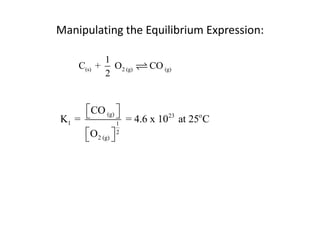

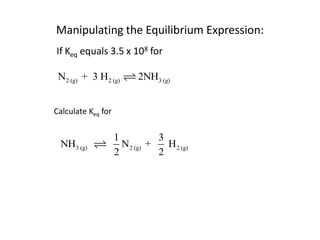
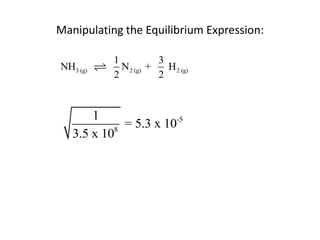
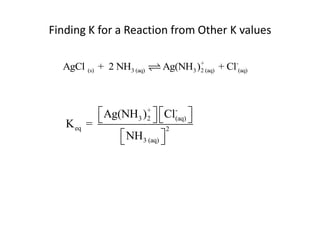
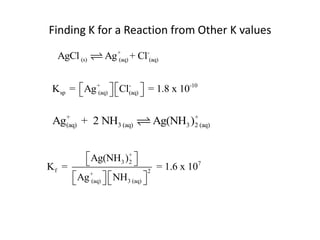
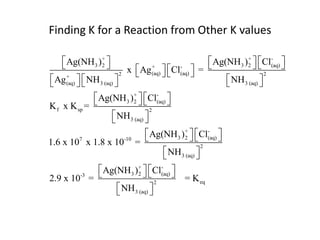
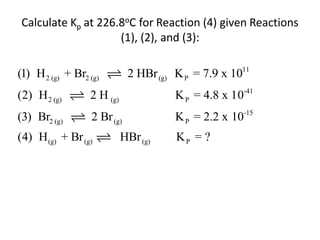
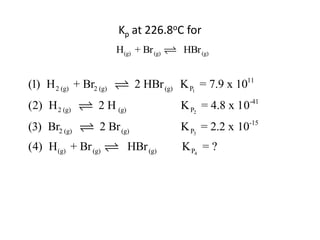

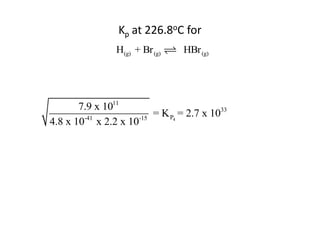

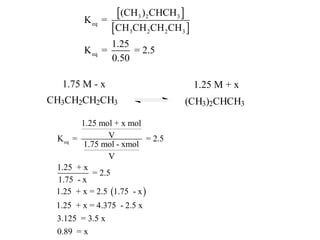
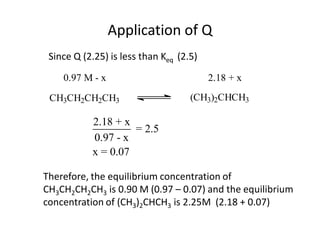
![Application of Q
For the following equilibrium:
CH3CH2CH2CH3 (CH3 )2CHCH3
Keq = 2.5 at 25oC is the reaction at equilibrium when
[CH3CH2CH2CH3] = 0.97 M and [(CH3)2CHCH3] = 2.18M?
If not, calculate the equilibrium concentrations.](https://image.slidesharecdn.com/gcchemicalequilibrium-110526013040-phpapp02/85/Gc-chemical-equilibrium-28-320.jpg)

![Another Application of Q
For the following equilibrium:
N2 (g) + O2 (g) 2 NO (g)
Keq = 1.7 x 10-3 is the reaction at equilibrium when
[N2] = 0.50 M , [O2] = 0.25 M, and [NO] = 0.0042 M?
If not, calculate the equilibrium concentrations.](https://image.slidesharecdn.com/gcchemicalequilibrium-110526013040-phpapp02/85/Gc-chemical-equilibrium-31-320.jpg)