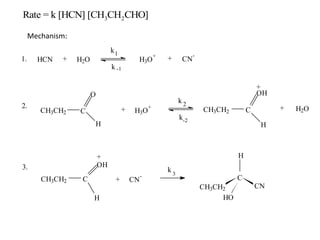
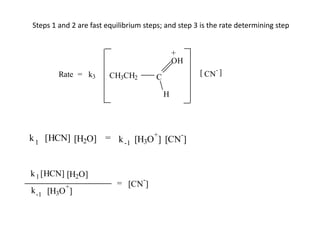
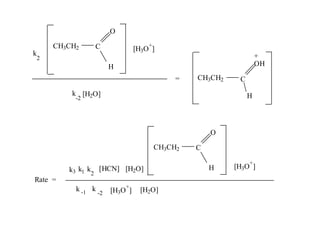
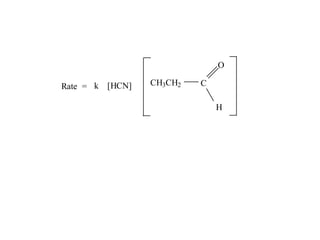
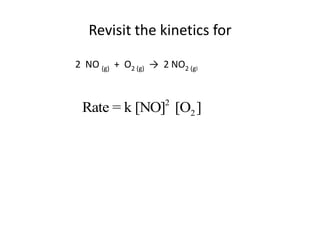
The document discusses chemical kinetics and provides information about:
- The factors that affect the speed of a chemical reaction, including concentration, temperature, and catalysts.
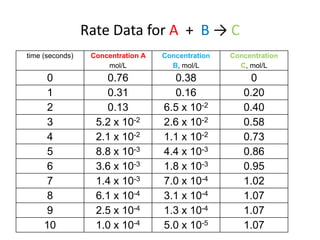
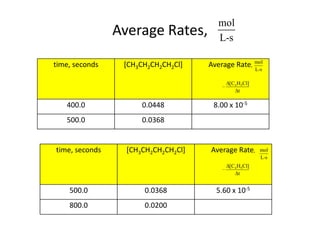
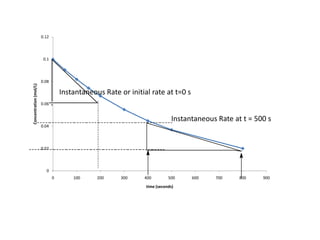
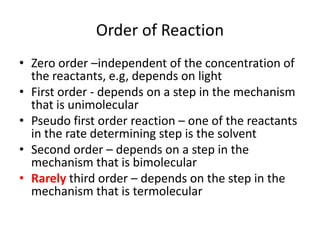
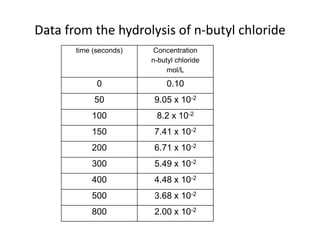
- How to determine the rate law, rate constant, order, and mechanism of reactions from experimental data.
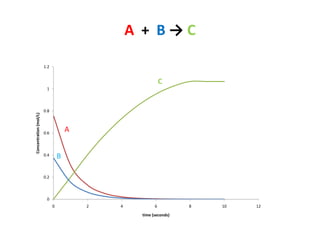
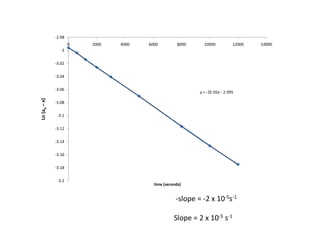
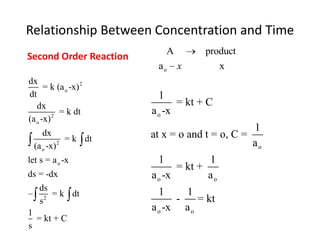
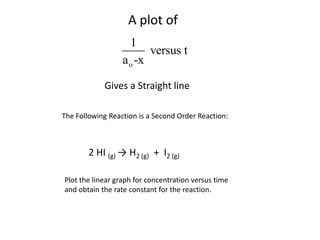
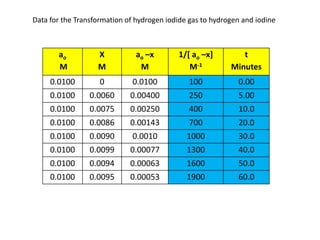
- The relationship between concentration and time for reactions of different orders (zero, first, and second order).
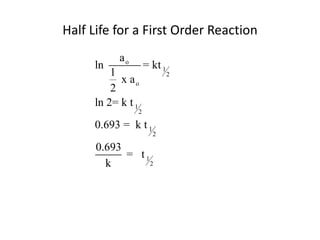
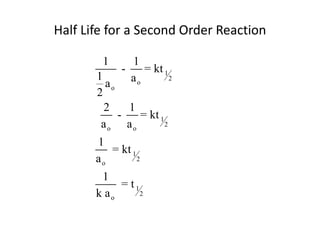
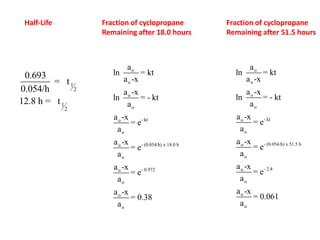
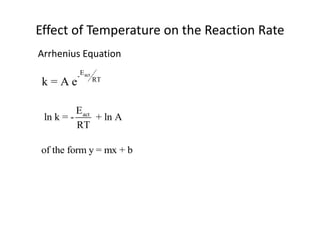
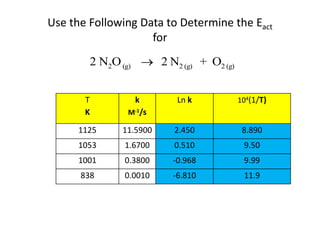
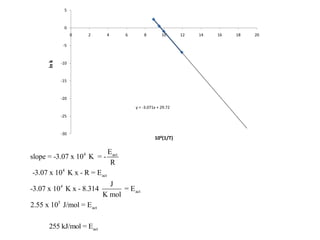
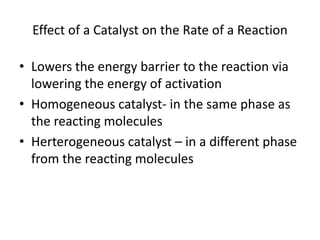
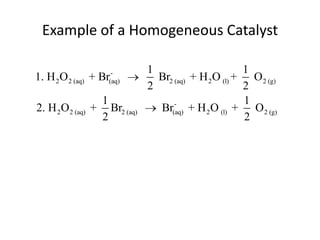
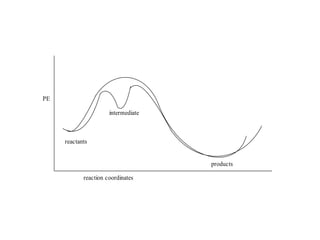
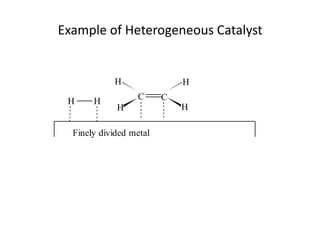
- How to calculate half-life, effect of temperature on reaction rate using the Arrhenius equation, and the role of homogeneous and heterogeneous catalysts.
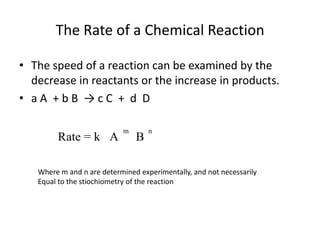
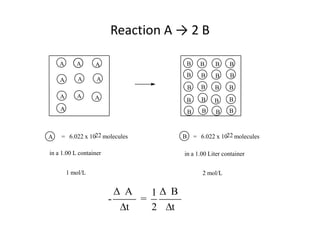
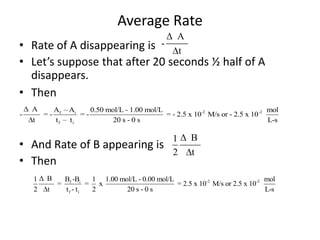
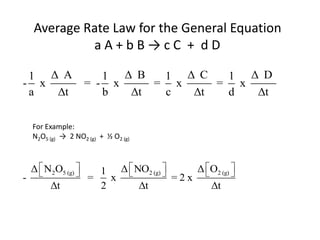

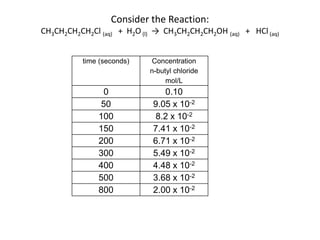
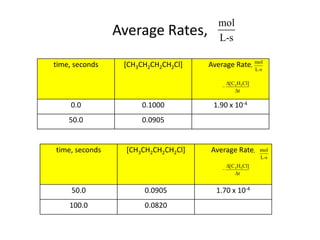
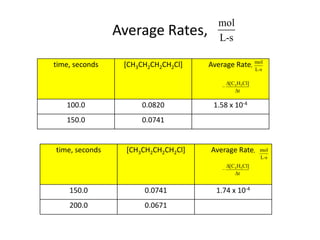
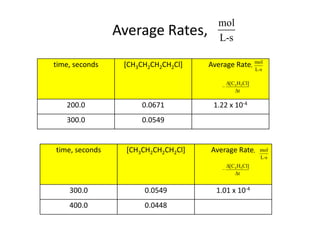

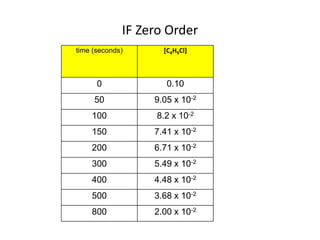
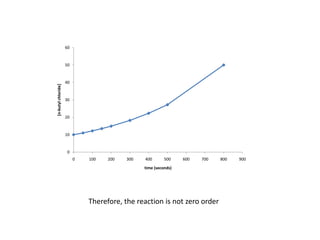
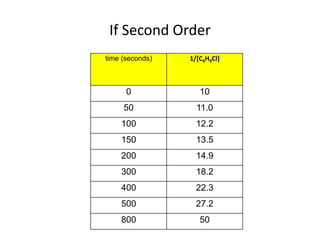
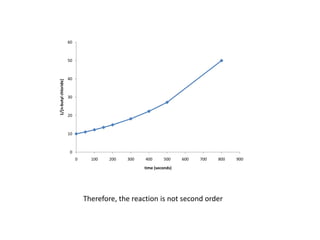
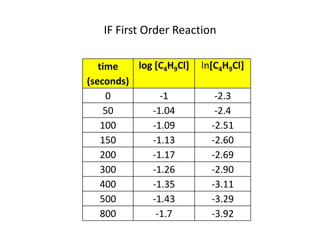
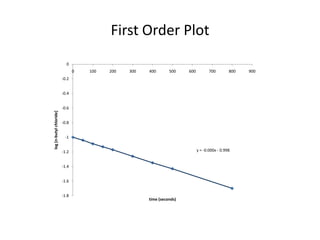
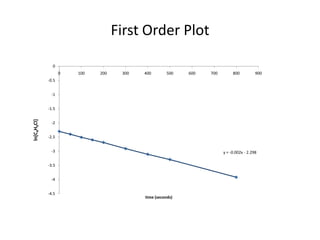
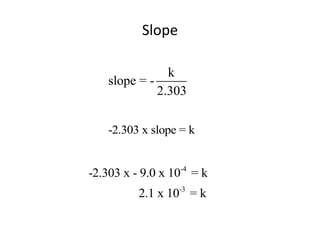
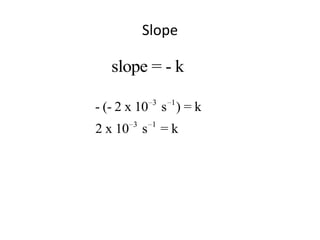
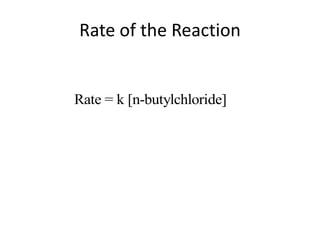
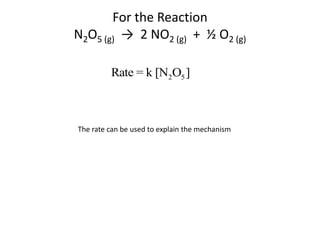
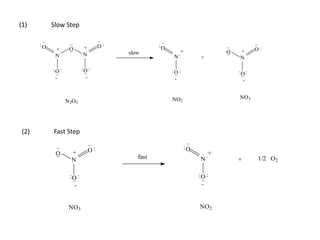
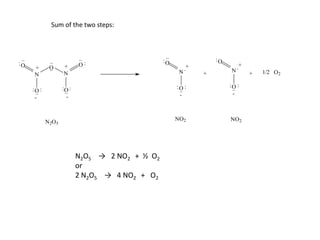
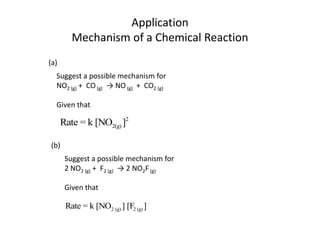
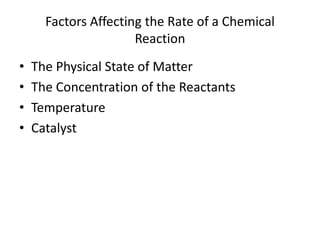

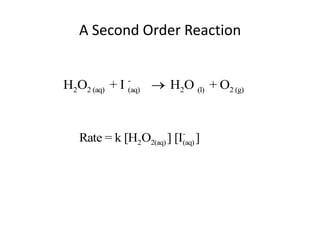

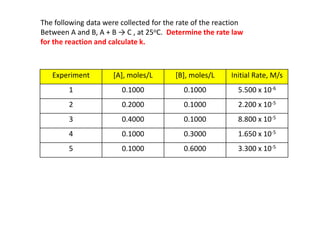
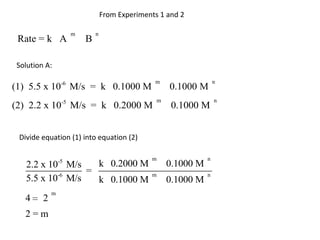

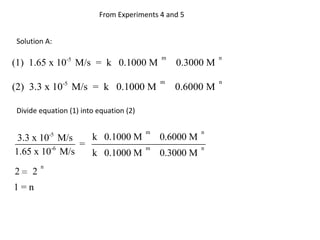

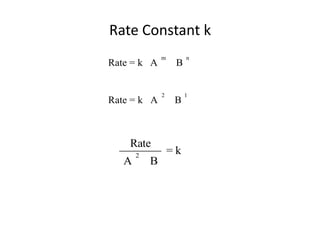
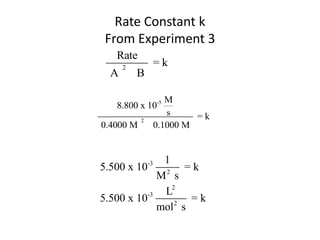
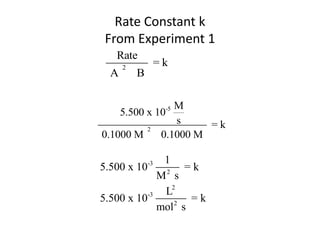
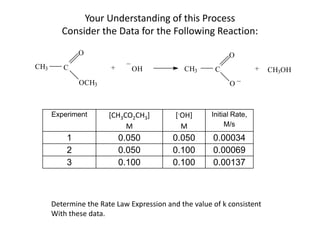
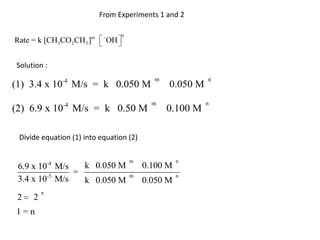
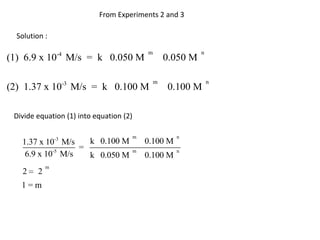
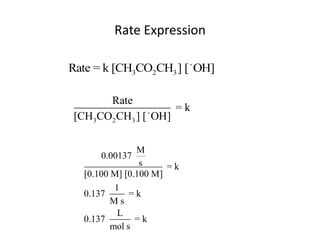
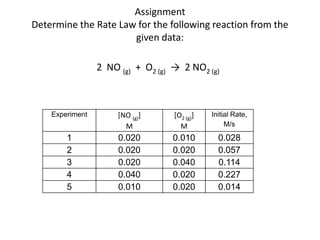
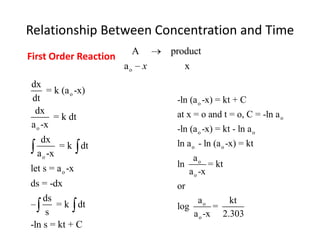
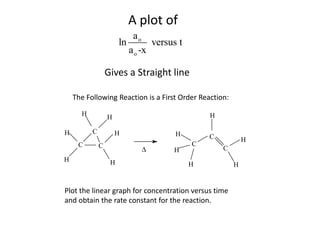
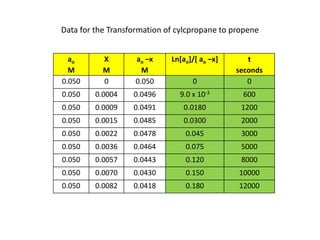
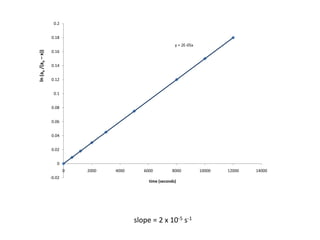
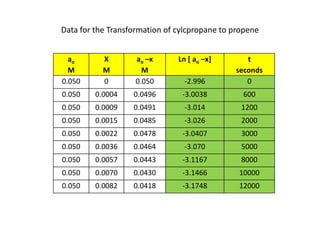
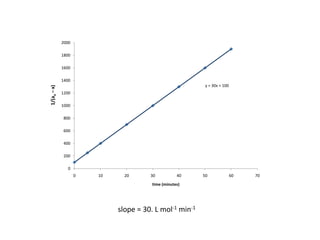
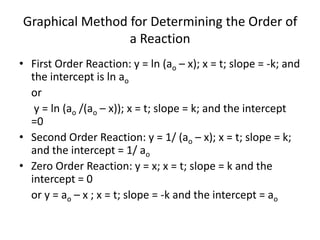
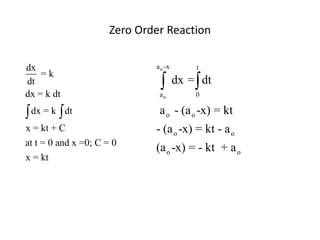
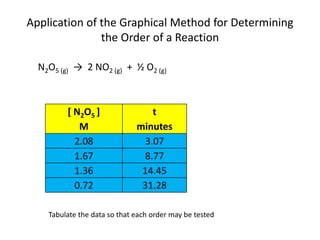
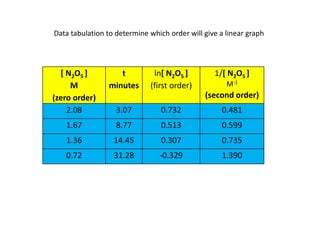
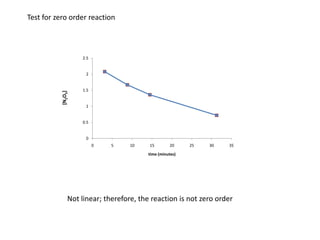
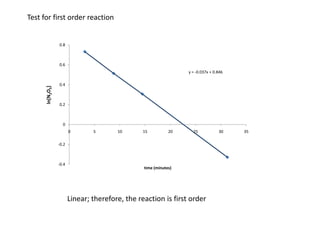
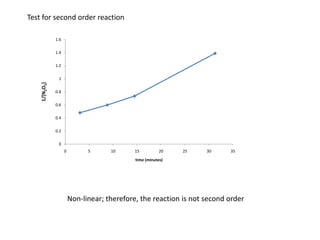
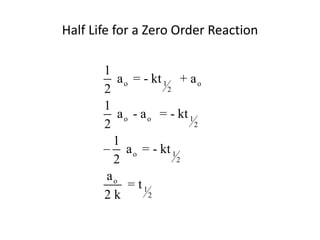

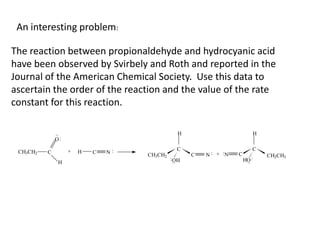
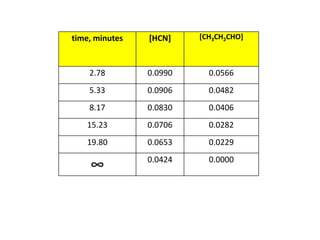
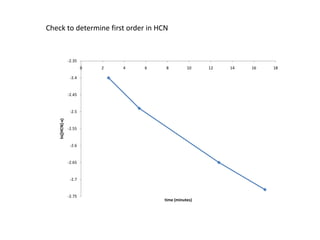
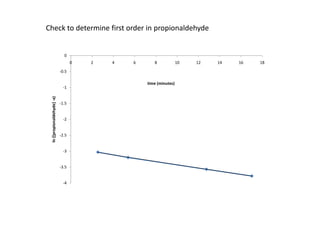
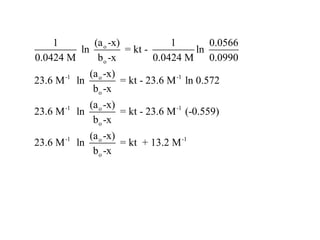
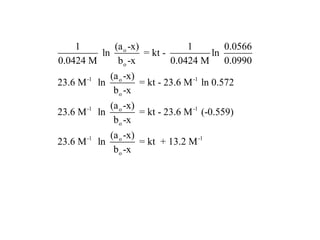
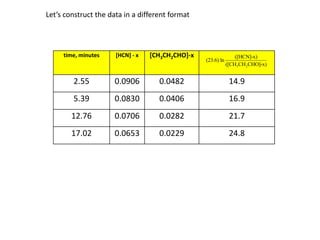
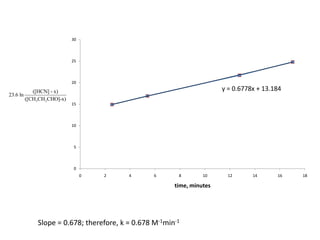
![So close; therefore, let’s take another approach. Let [HCN] = ao and [propionaldehyde] = boThen,](https://image.slidesharecdn.com/gcchemicalkinetics-110510150117-phpapp02/85/GC-Chemical-Kinetics-82-320.jpg)