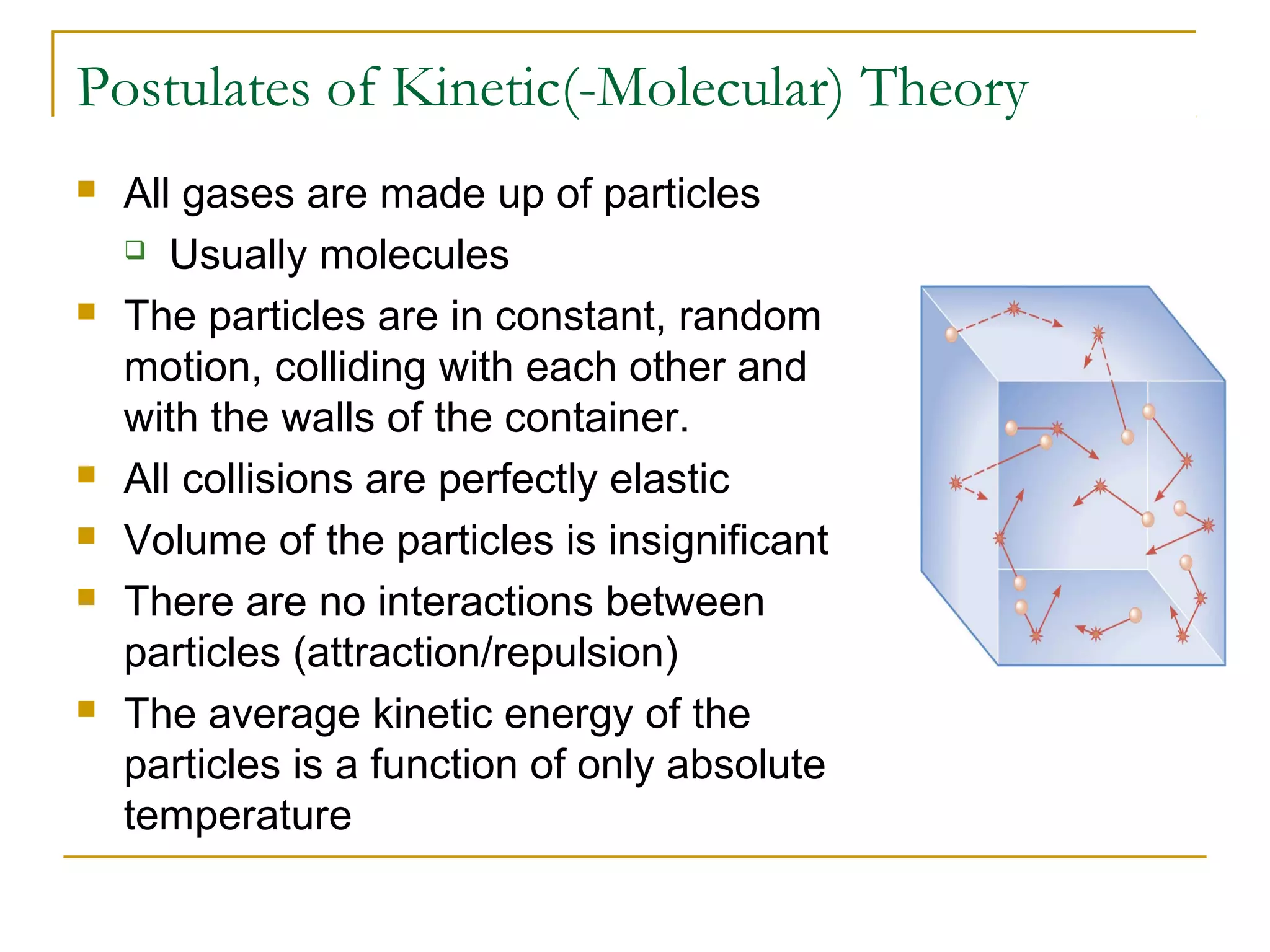
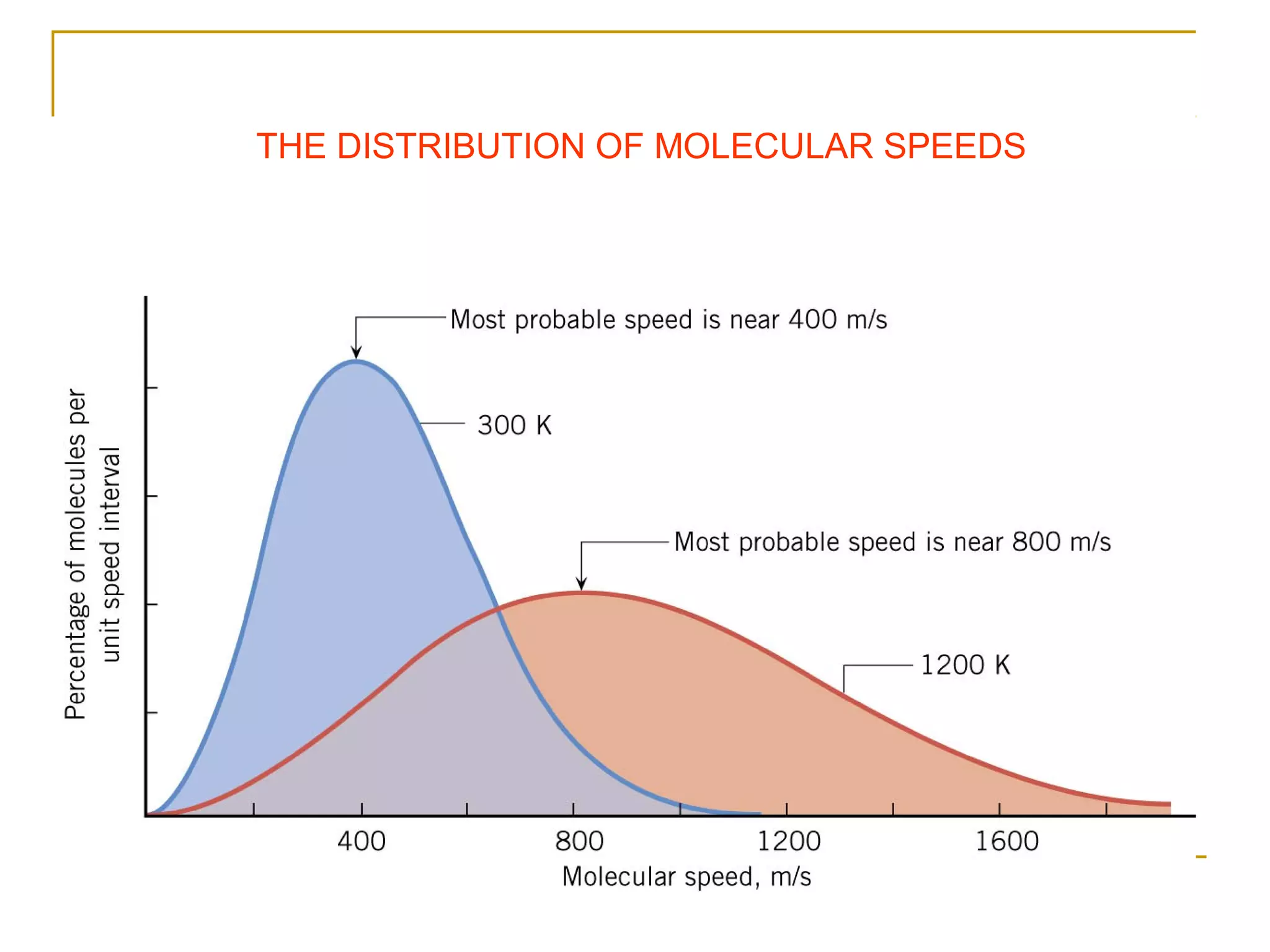
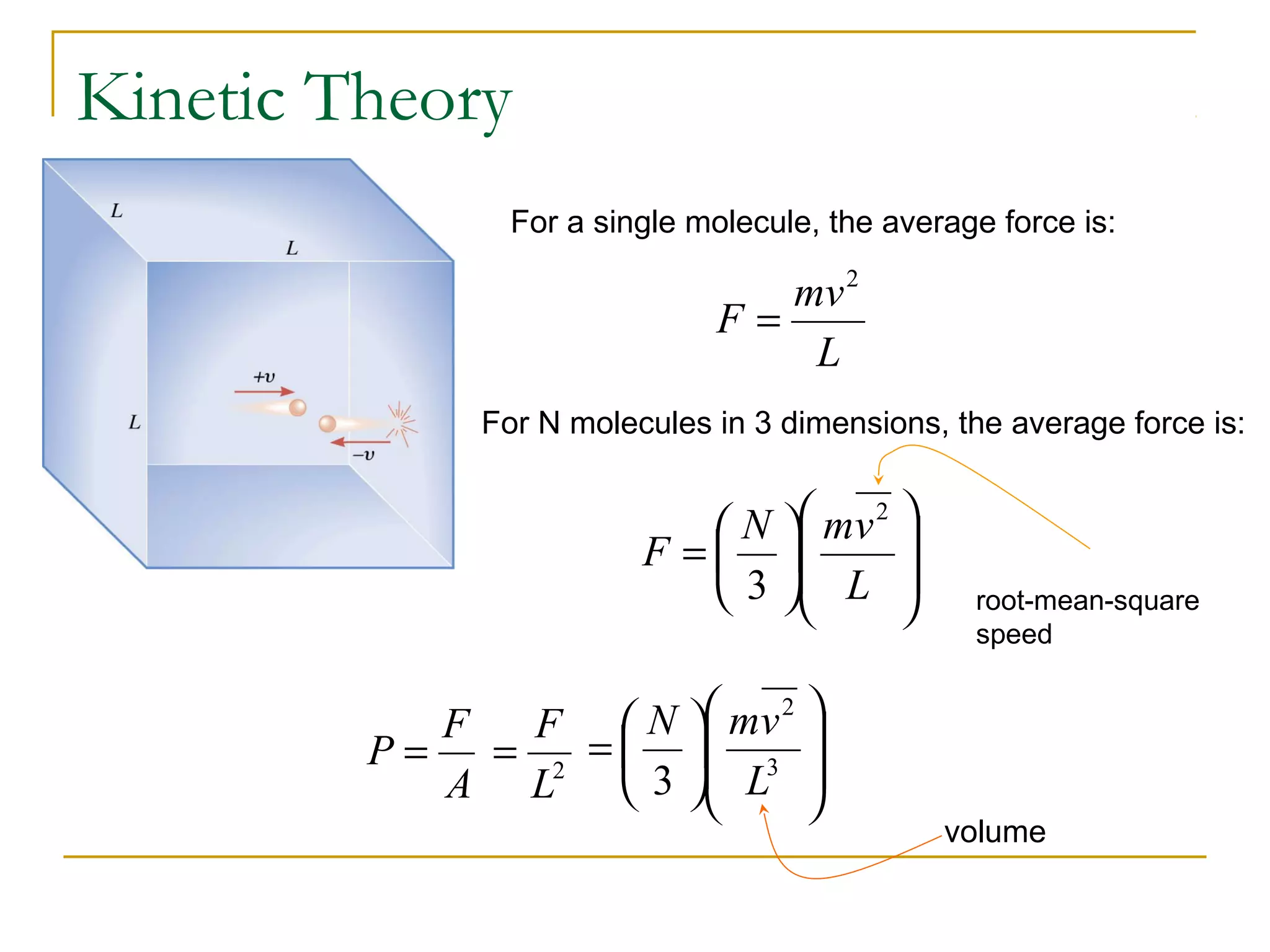
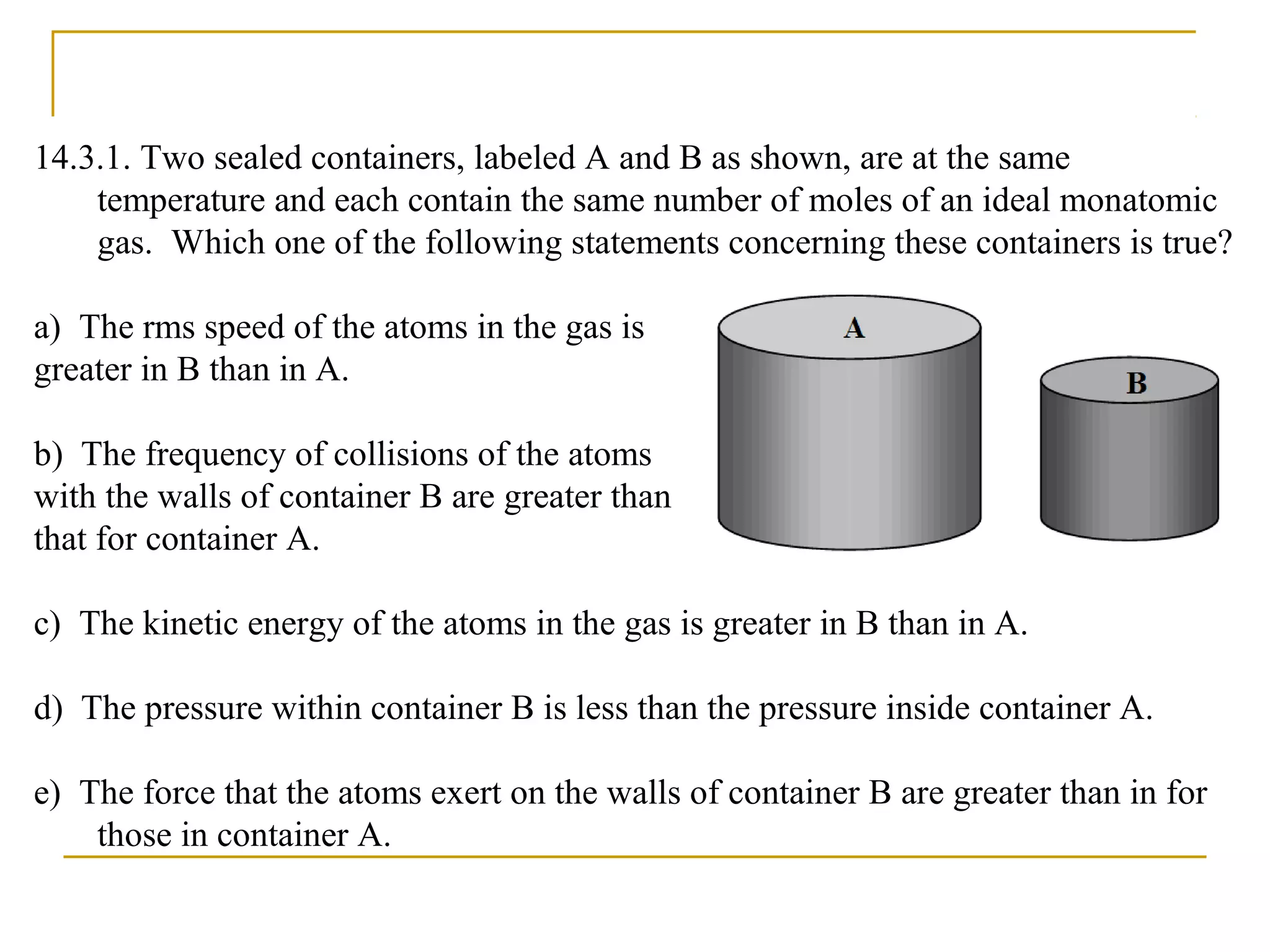
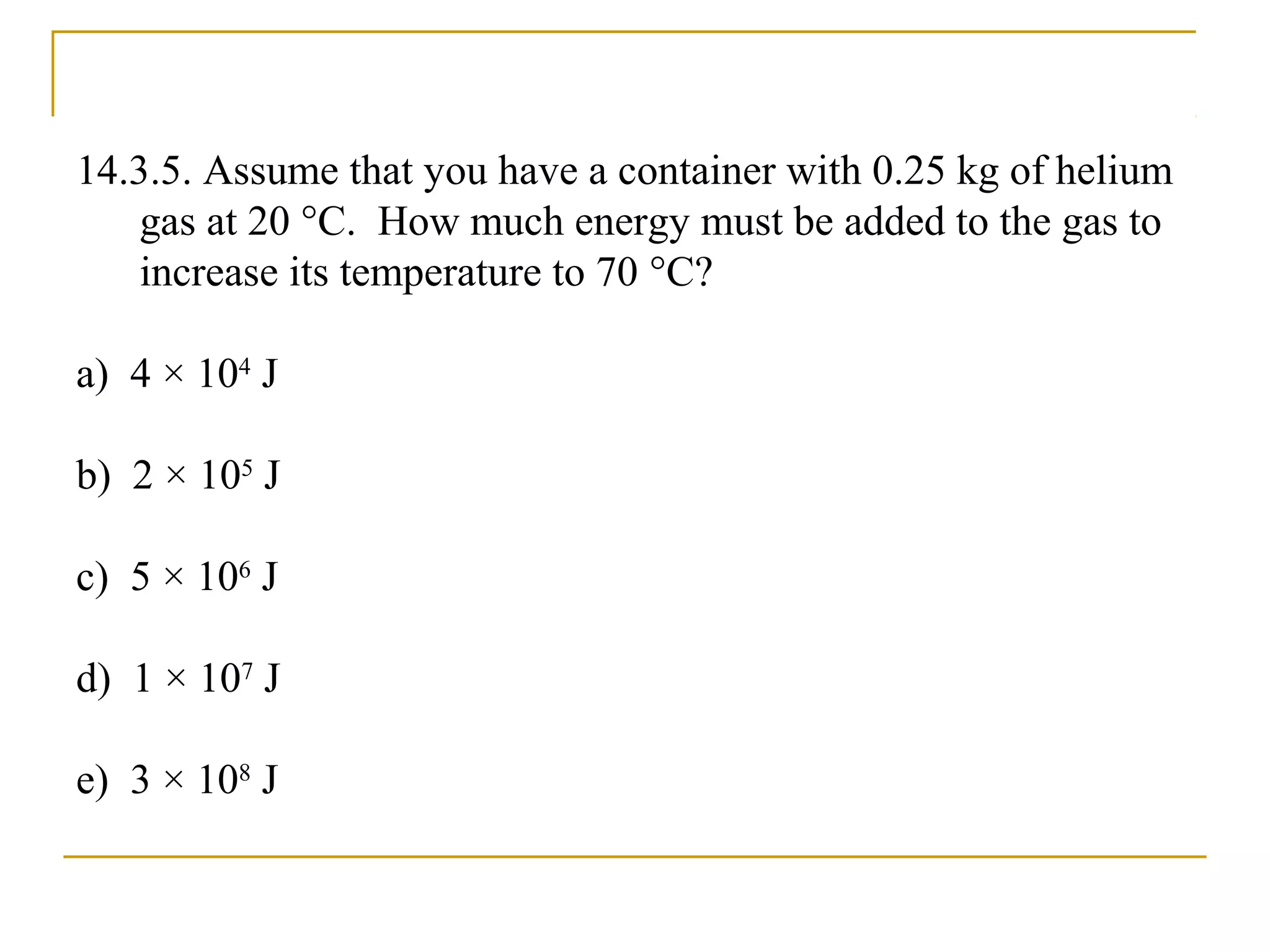
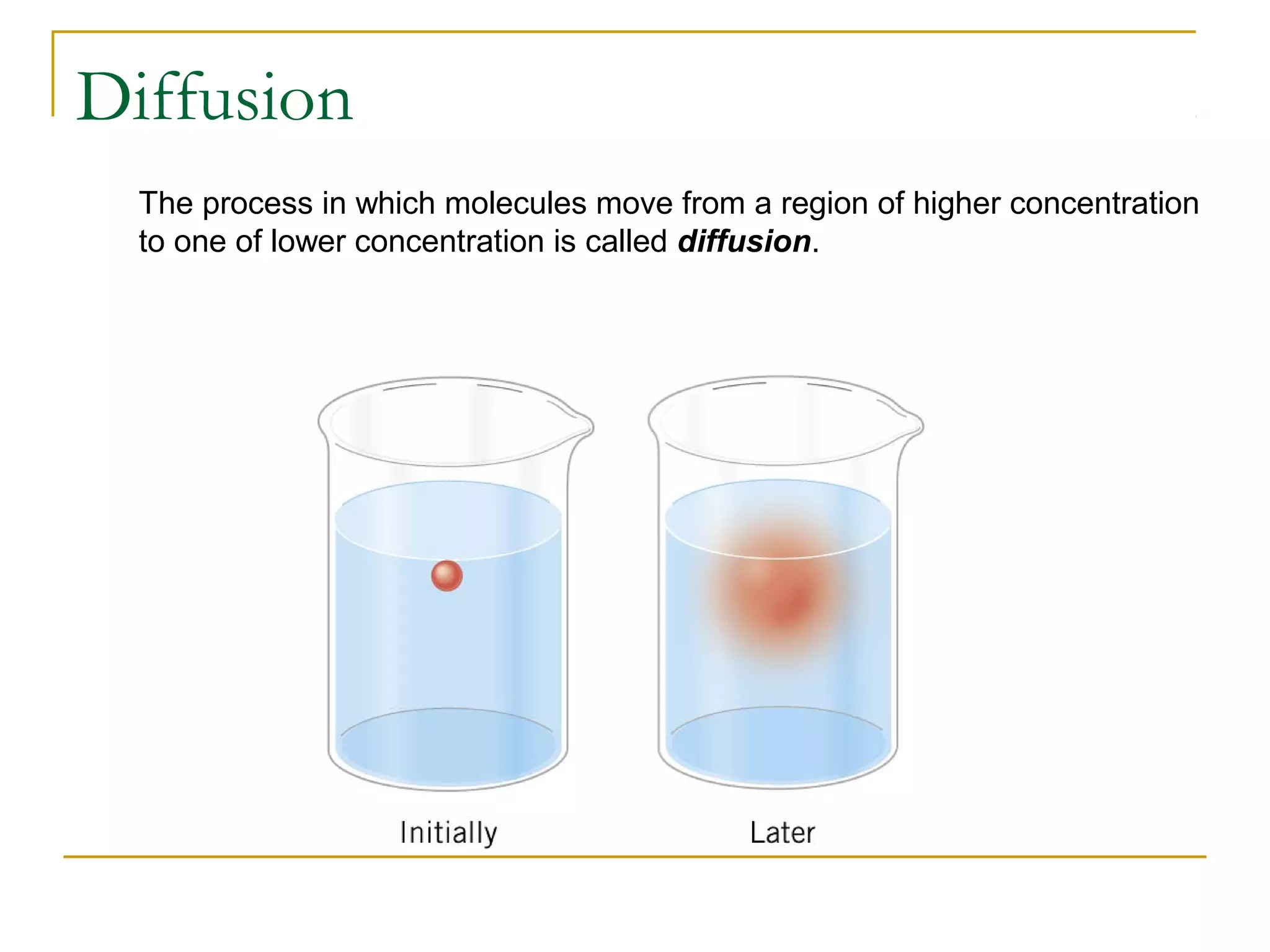
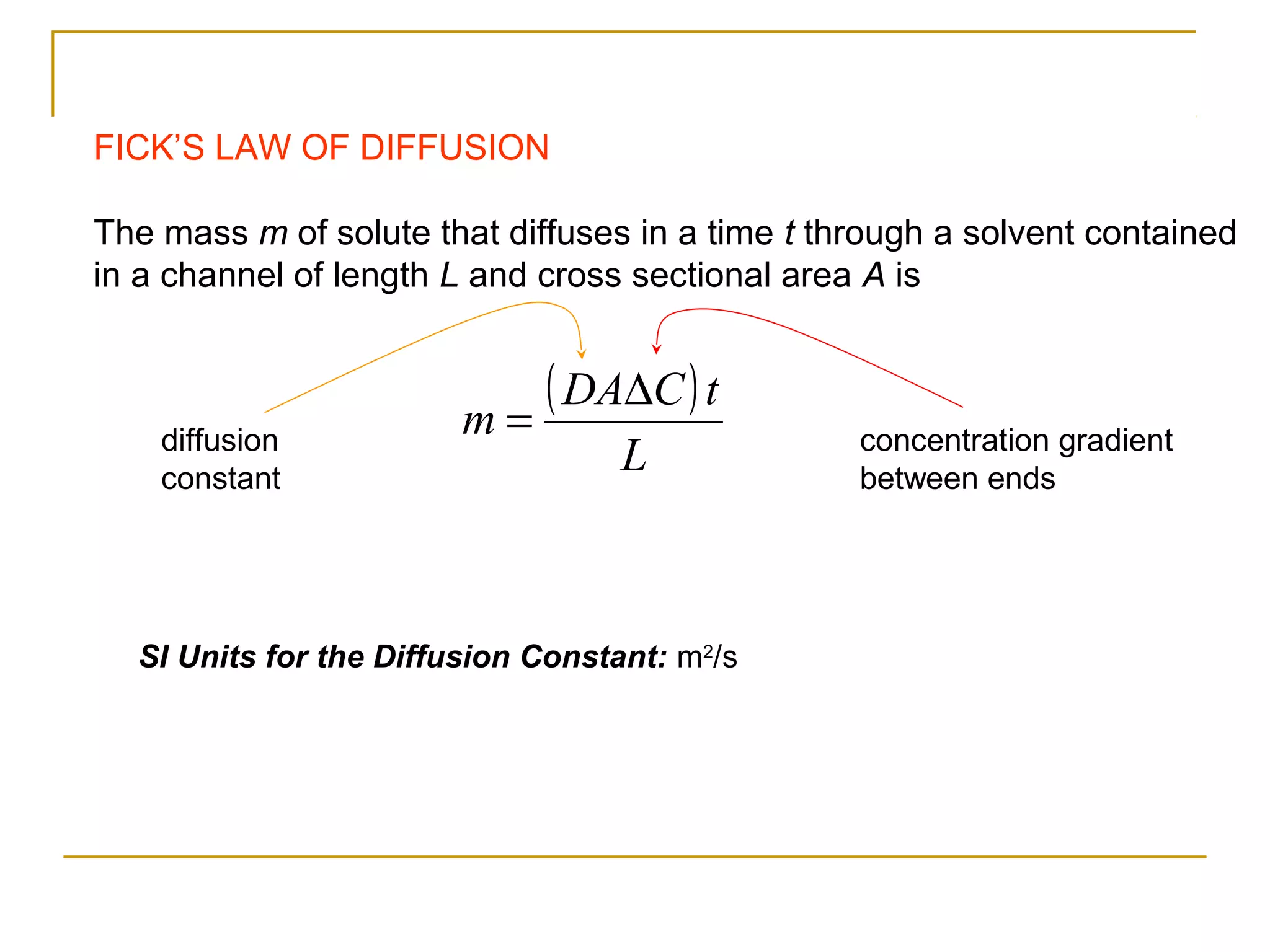
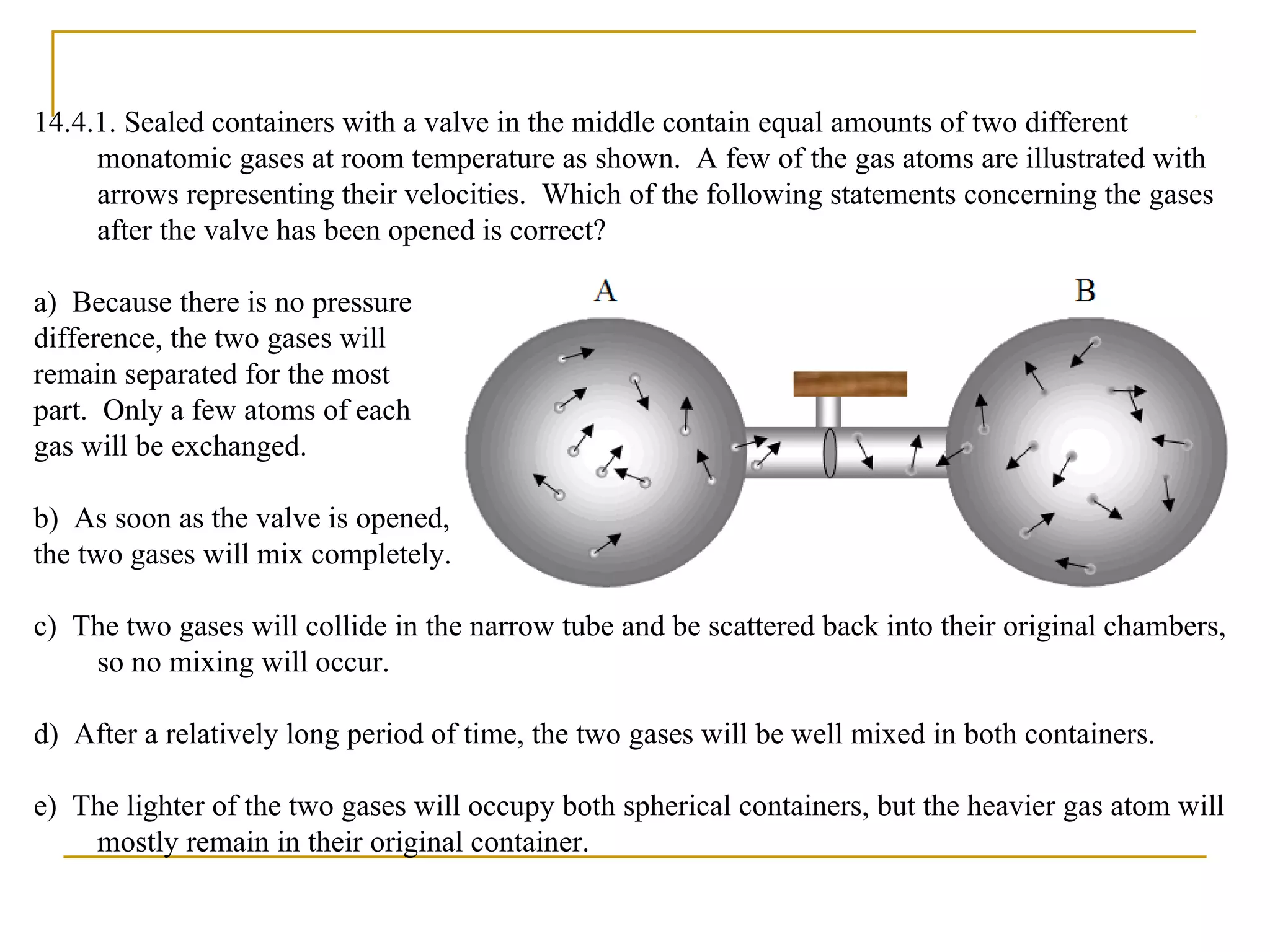
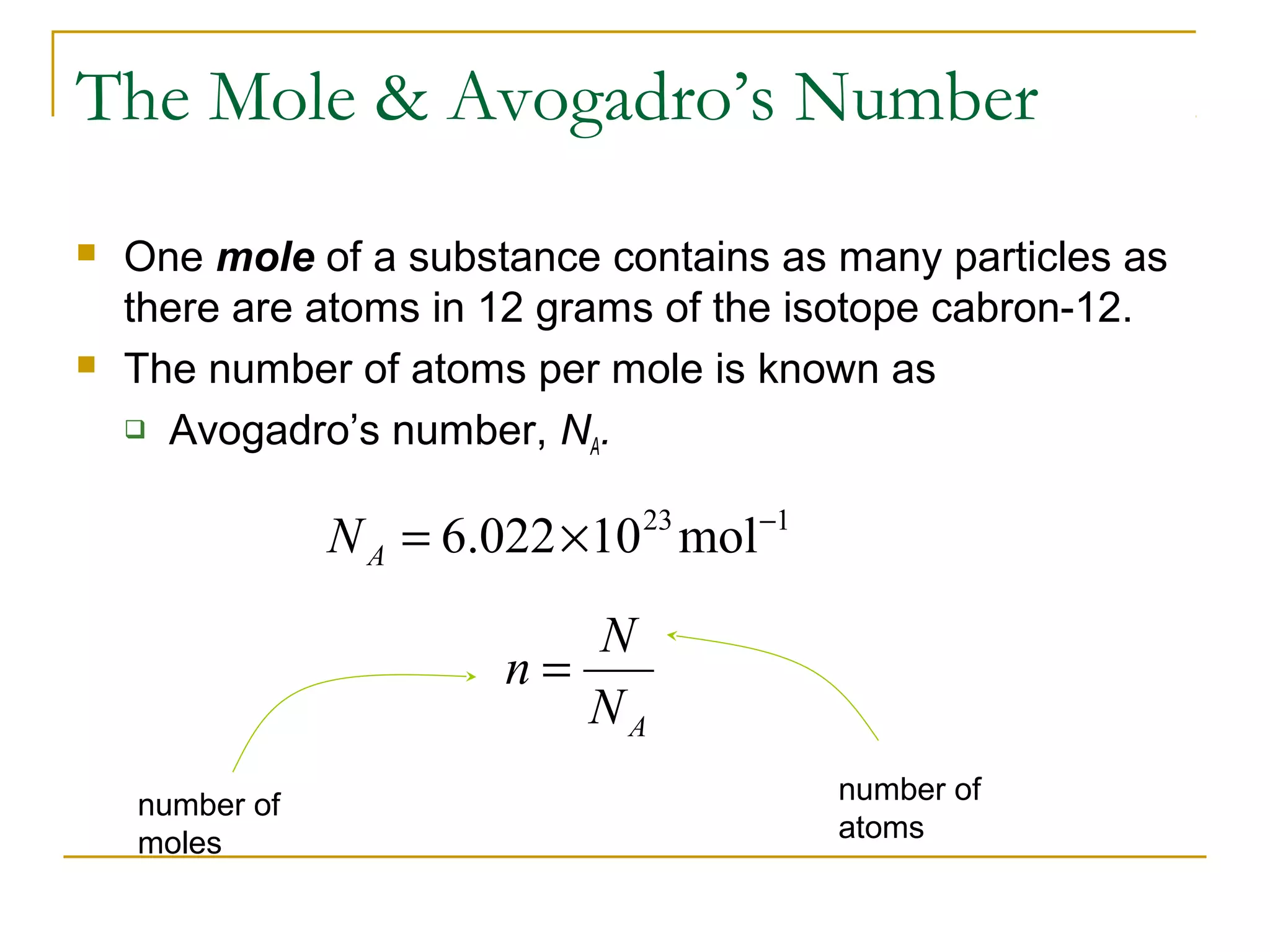
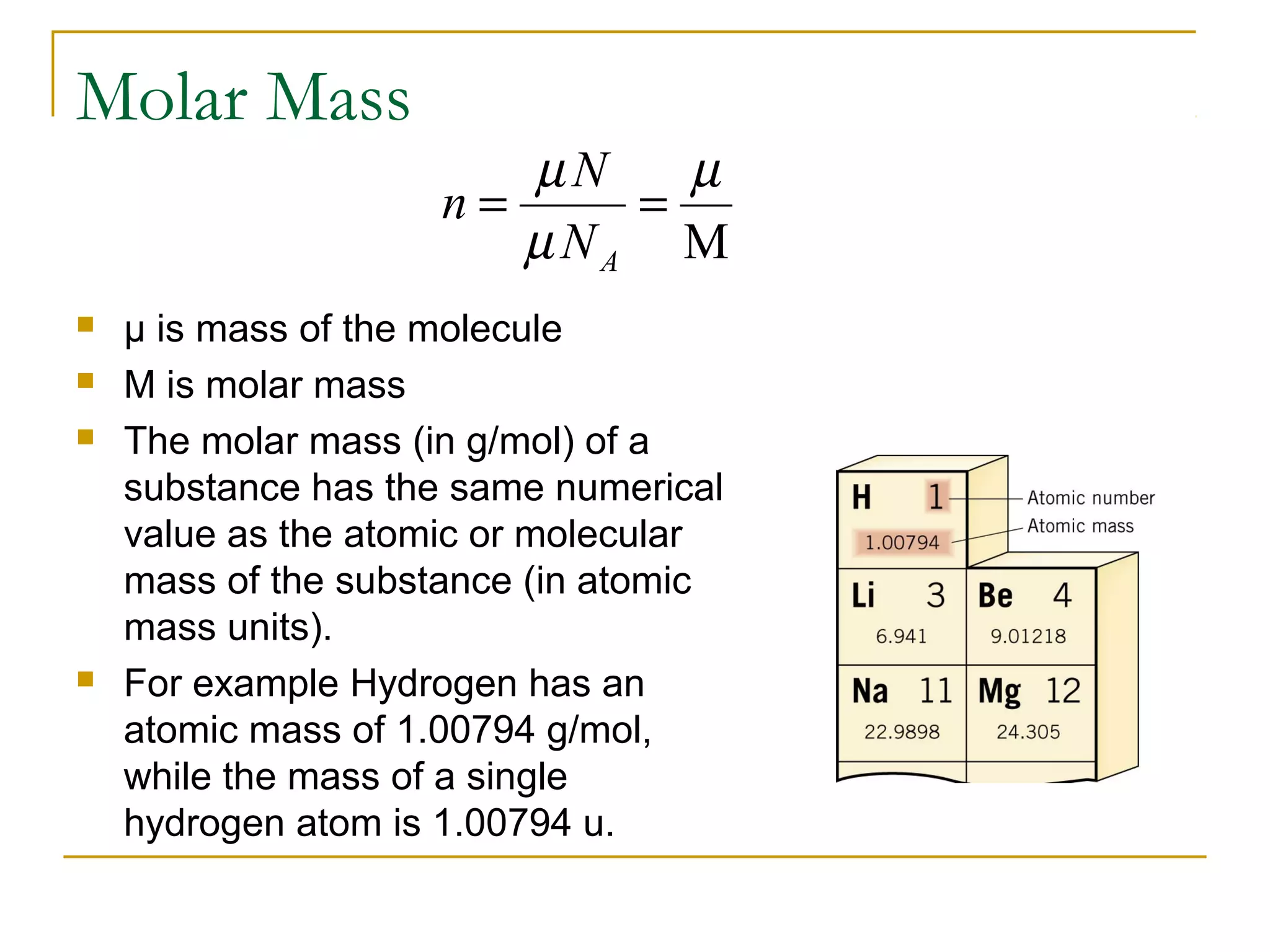
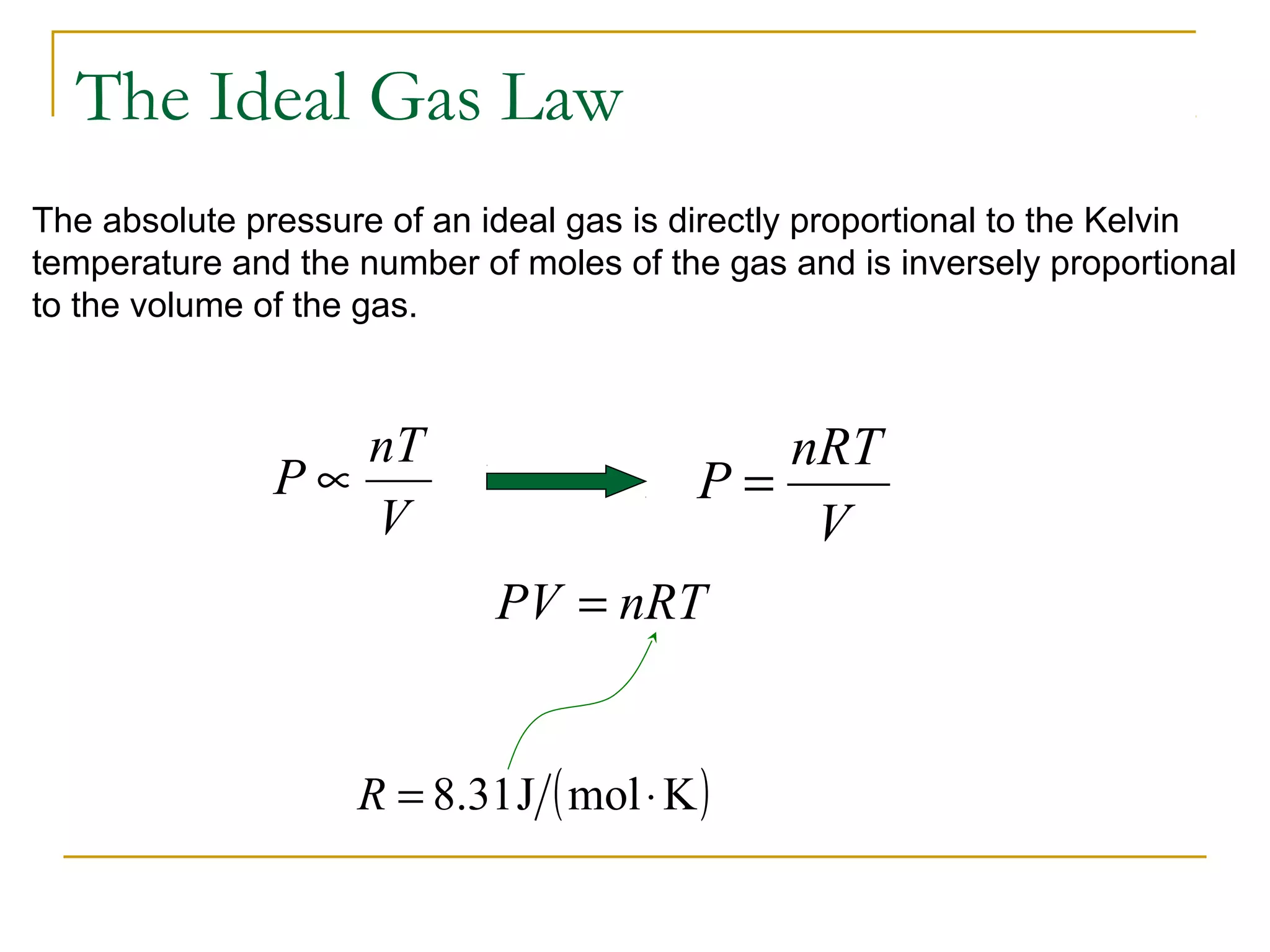
The document summarizes key concepts from Chapter 14 on the ideal gas law and kinetic theory. Section 1 discusses molecular mass, the mole, and Avogadro's number. Section 2 covers the ideal gas law and how pressure, volume, temperature, and moles are related. Section 3 introduces the kinetic theory model, which describes gases as large numbers of constantly moving particles and explains gas properties and behaviors in terms of particle collisions and kinetic energy.



![Example 1 The Hope Diamond and the Rosser Reeves Ruby
The Hope diamond (44.5 carats) is almost pure carbon. The Rosser
Reeves ruby (138 carats) is primarily aluminum oxide (Al2O3). One
carat is equivalent to a mass of 0.200 g. Determine (a) the number of
carbon atoms in the Hope diamond and (b) the number of Al2O3
molecules in the ruby.
M
µ
=n(a)
(b) ( ) ( ) ( )[ ]
molg96.101
carat1g200.0carats138
=
AnNN =
AnNN =
( ) ( ) ( )[ ]
molg011.12
carat1g200.0carats5.44
= mol741.0=
( )( )123
mol10022.6mol741.0 −
×= atoms1046.4 23
×=
M
µ
=n
( ) ( )
99.15398.262 +
mol271.0=
( )( )123
mol10022.6mol271.0 −
×= atoms1063.1 23
×=](https://image.slidesharecdn.com/ch14iglandkmtonline-101031113719-phpapp01/75/Ch-14-Ideal-Gas-Law-Kinetic-Theory-8-2048.jpg)




![Example 2 Oxygen in the Lungs
In the lungs, the respiratory membrane separates tiny sacs of air
(pressure 1.00x105
Pa) from the blood in the capillaries. These sacs
are called alveoli. The average radius of the alveoli is 0.125 mm, and
the air inside contains 14% oxygen. Assuming that the air behaves as
an ideal gas at 310K, find the number of oxygen molecules in one of
these sacs.
TNkPV B=
Tk
PV
N
B
=
( )( ) 2
1314
Oofmolecules107.214.0109.1 ×=×=N
( ) ( )[ ]
( )( )K310KJ1038.1
m10125.0Pa1000.1
23
33
3
45
−
−
×
××
=
π
airofmolecules109.1 14
×=N](https://image.slidesharecdn.com/ch14iglandkmtonline-101031113719-phpapp01/75/Ch-14-Ideal-Gas-Law-Kinetic-Theory-18-2048.jpg)