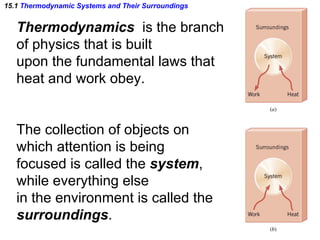
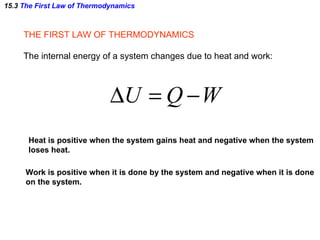
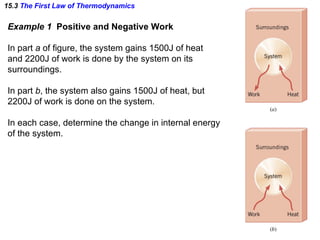
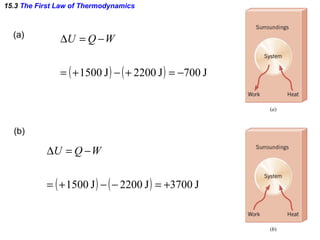
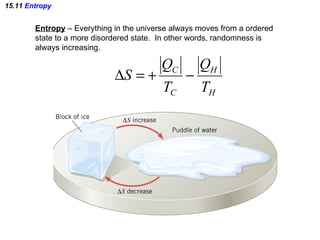
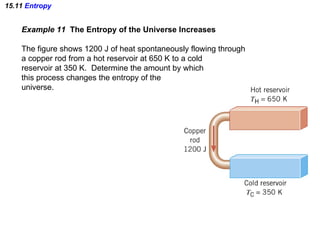
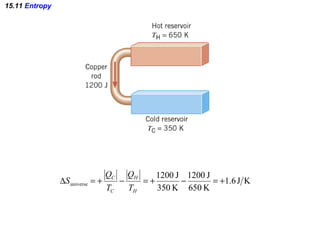
The document discusses the key concepts of thermodynamics including:
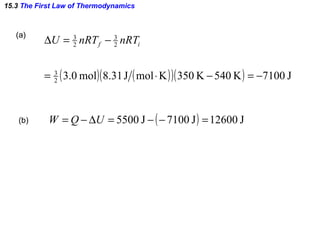
1) The first law of thermodynamics which states that the change in internal energy of a system is equal to the heat supplied plus work done.
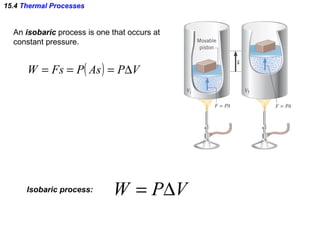
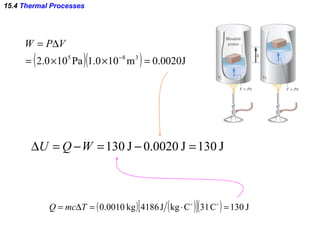
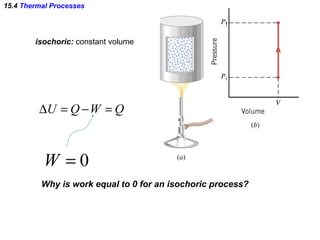
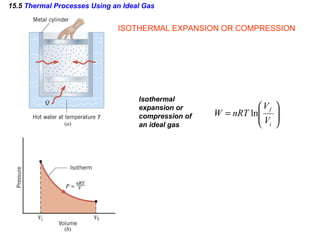
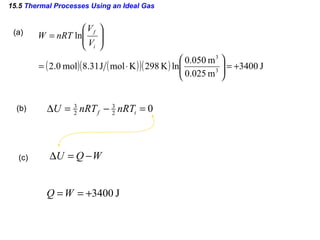
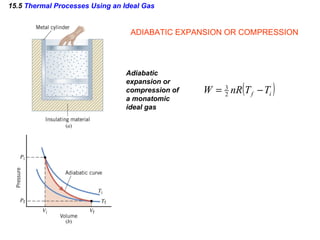
2) The four main types of thermal processes - isobaric, isochoric, isothermal, and adiabatic - which involve constant pressure, volume, temperature, and no heat transfer respectively.
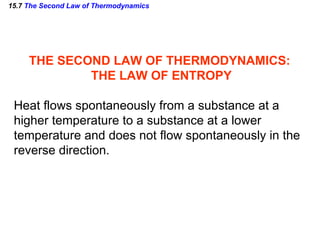
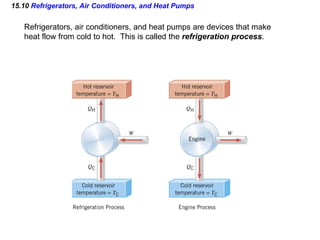
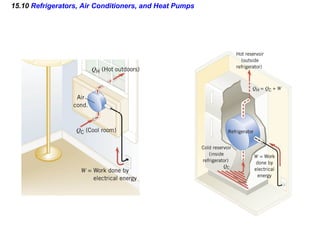
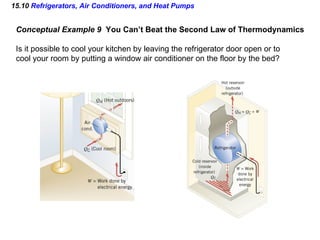
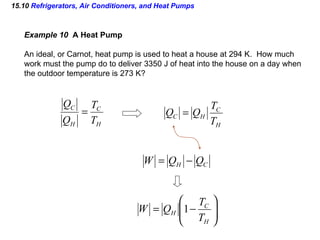
3) The second law of thermodynamics which states that heat spontaneously flows from hot to cold and not vice versa.
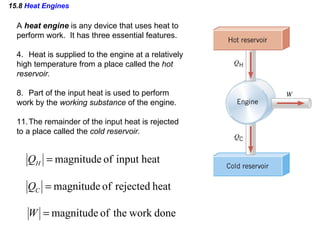
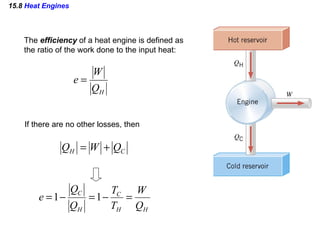
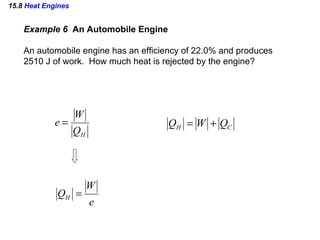
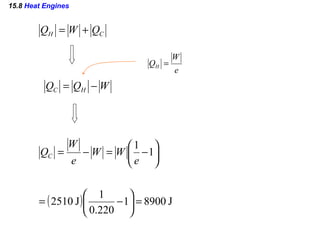
4) Heat engines which use heat to perform work and have an efficiency defined as the ratio of work done to input heat.