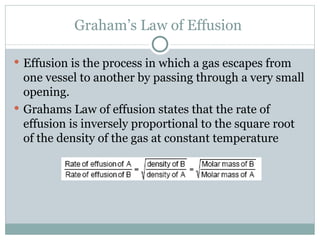
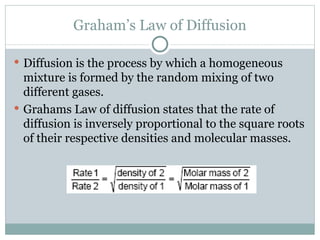
This document provides an overview of key concepts in gases, including:
1. Gases are composed of particles that move randomly and have negligible volume compared to their container. Gas pressure results from particle collisions with container walls.
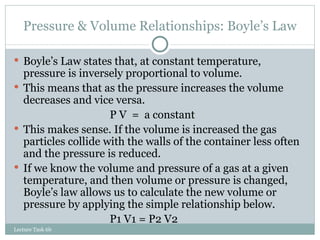
2. Boyle's, Charles', Gay-Lussac's, and Avogadro's laws describe relationships between gas properties like pressure, volume, temperature, and moles.
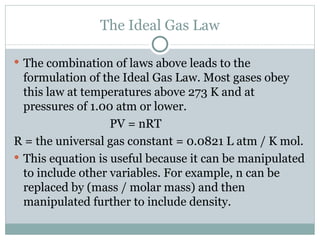
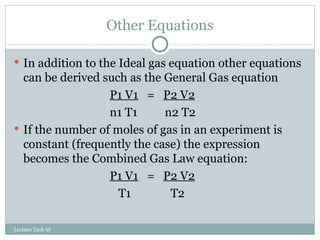
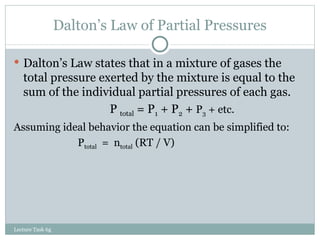
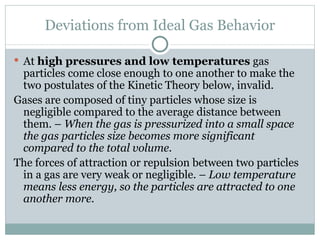
3. The ideal gas law combines these relationships, though gases deviate from ideal behavior at high pressures or low temperatures.