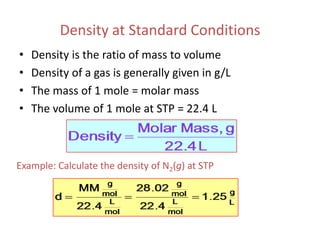
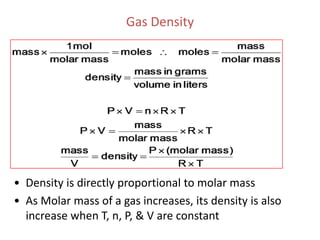
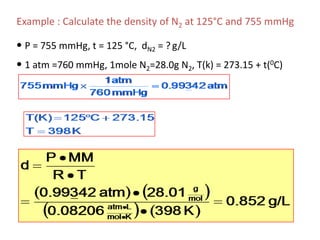
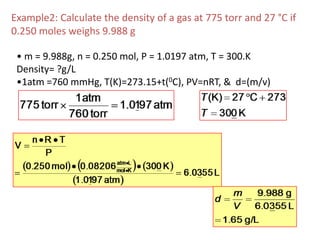
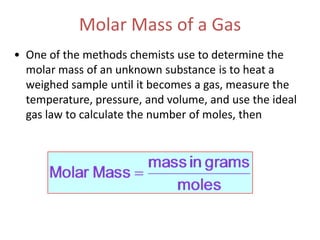
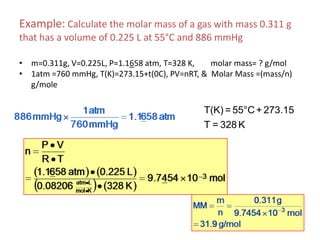
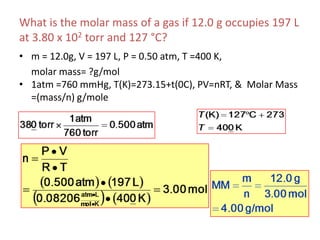
1) The document discusses the ideal gas law (PV=nRT) and its applications in calculating things like moles of gas, volume at different conditions, density, and molar mass.
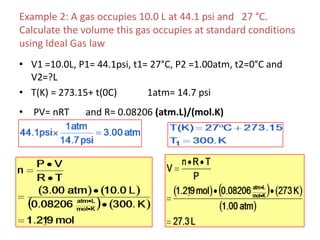
2) Key variables in the ideal gas law are defined such as pressure (P), volume (V), moles of gas (n), temperature (T), and the gas constant (R).
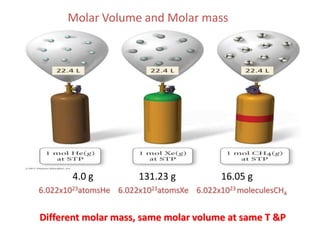
3) Standard temperature and pressure conditions are defined as 0°C and 1 atmosphere, where the molar volume of a gas is 22.4 L.
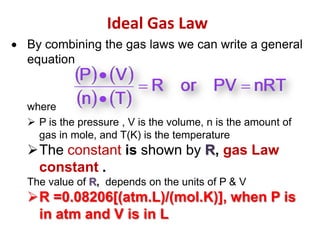
![The constant is shown byR, gas Law constant . The value of R, depends on the units of P & VR =0.08206[(atm.L)/(mol.K)], when P is in atm and V is in LExample: How many moles of gas are in a basketball with total pressure 24.3 psi, volume of 3.24 L at 25°C?V = 3.24 L, P = 24.3 psi, t = 25 °C and n=? Mol](https://image.slidesharecdn.com/idealgaslaw-practicemccpot-111011123153-phpapp02/85/Ideal-gas-law-practice-mccpot-3-320.jpg)