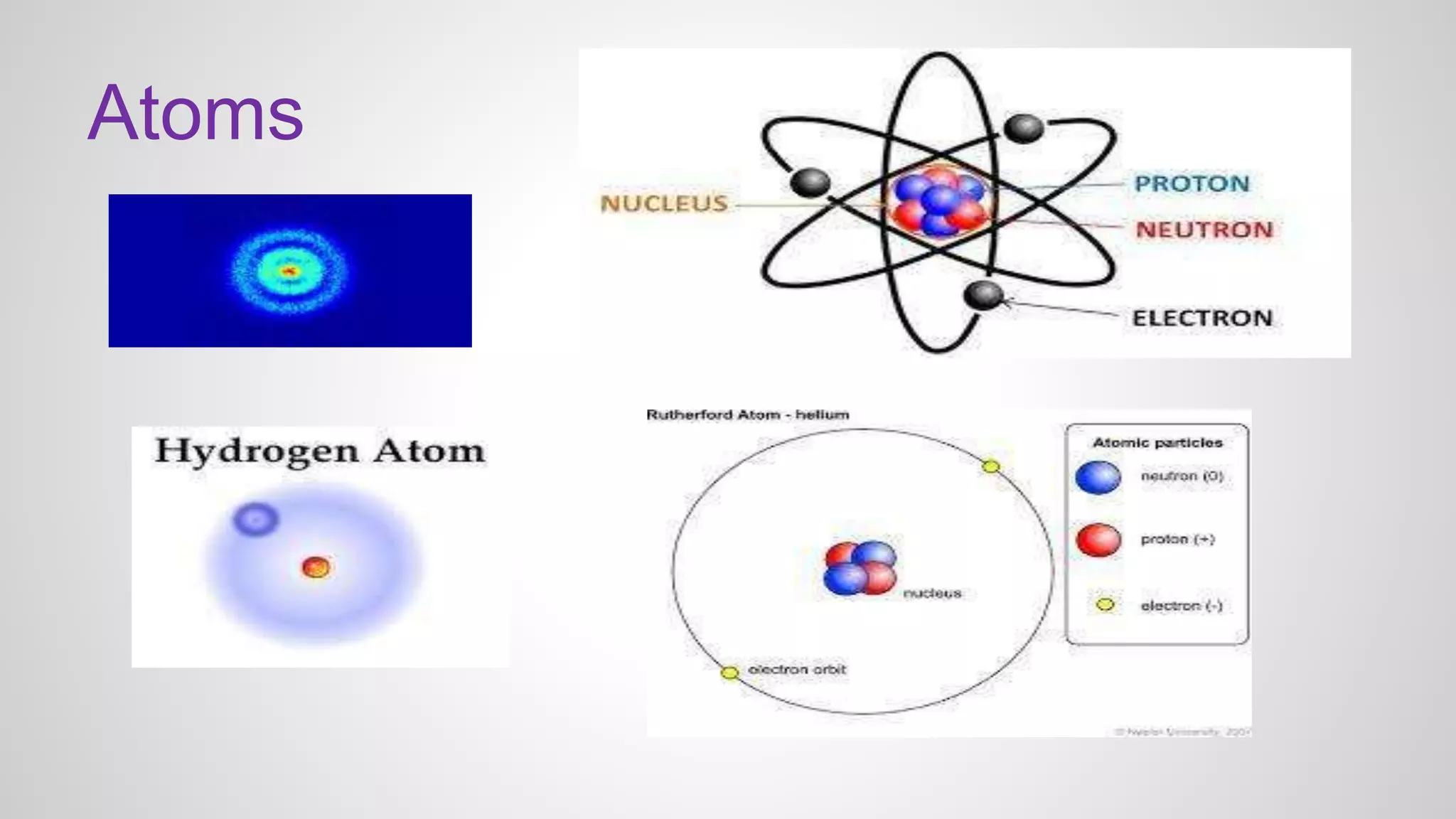
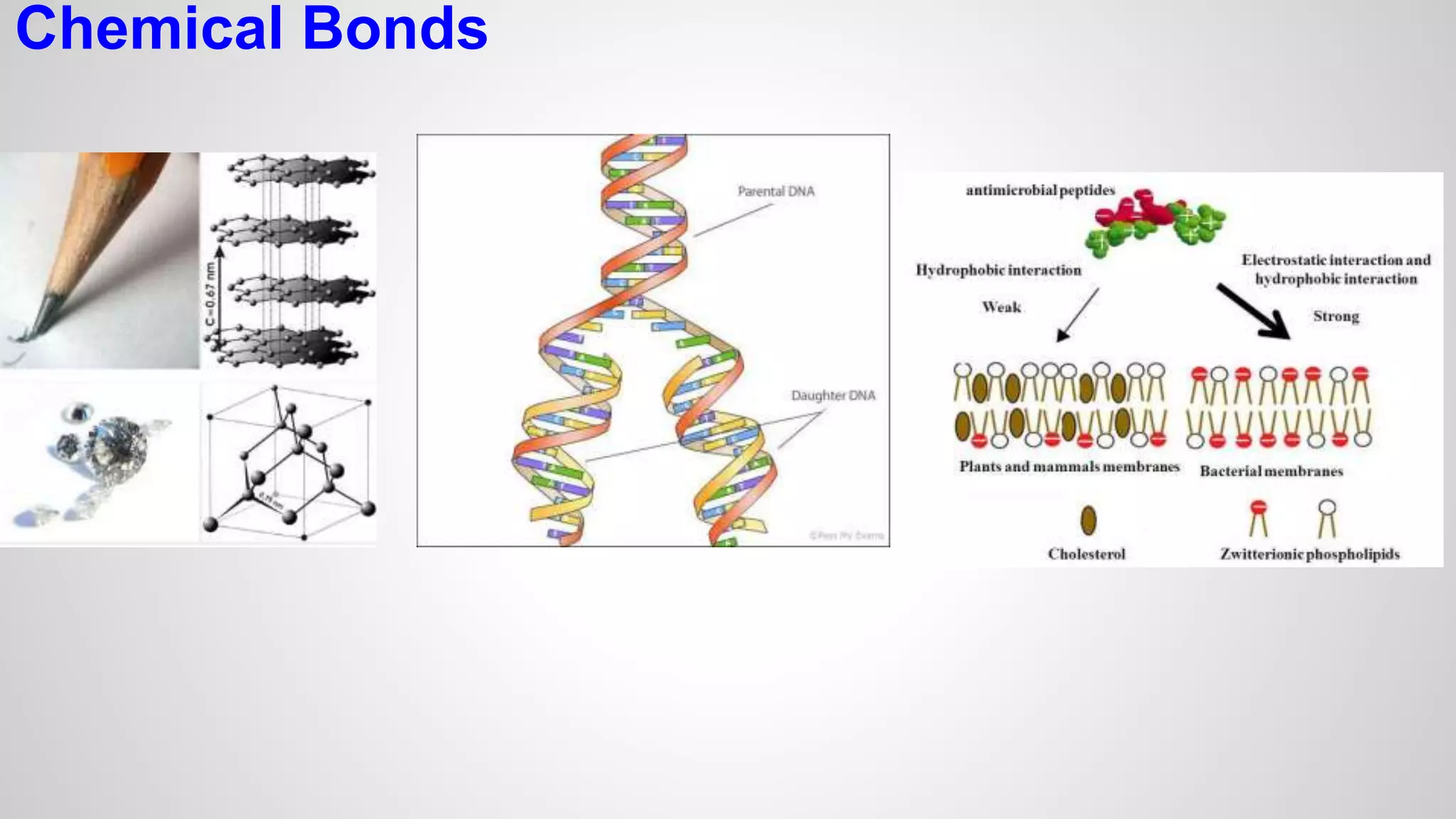
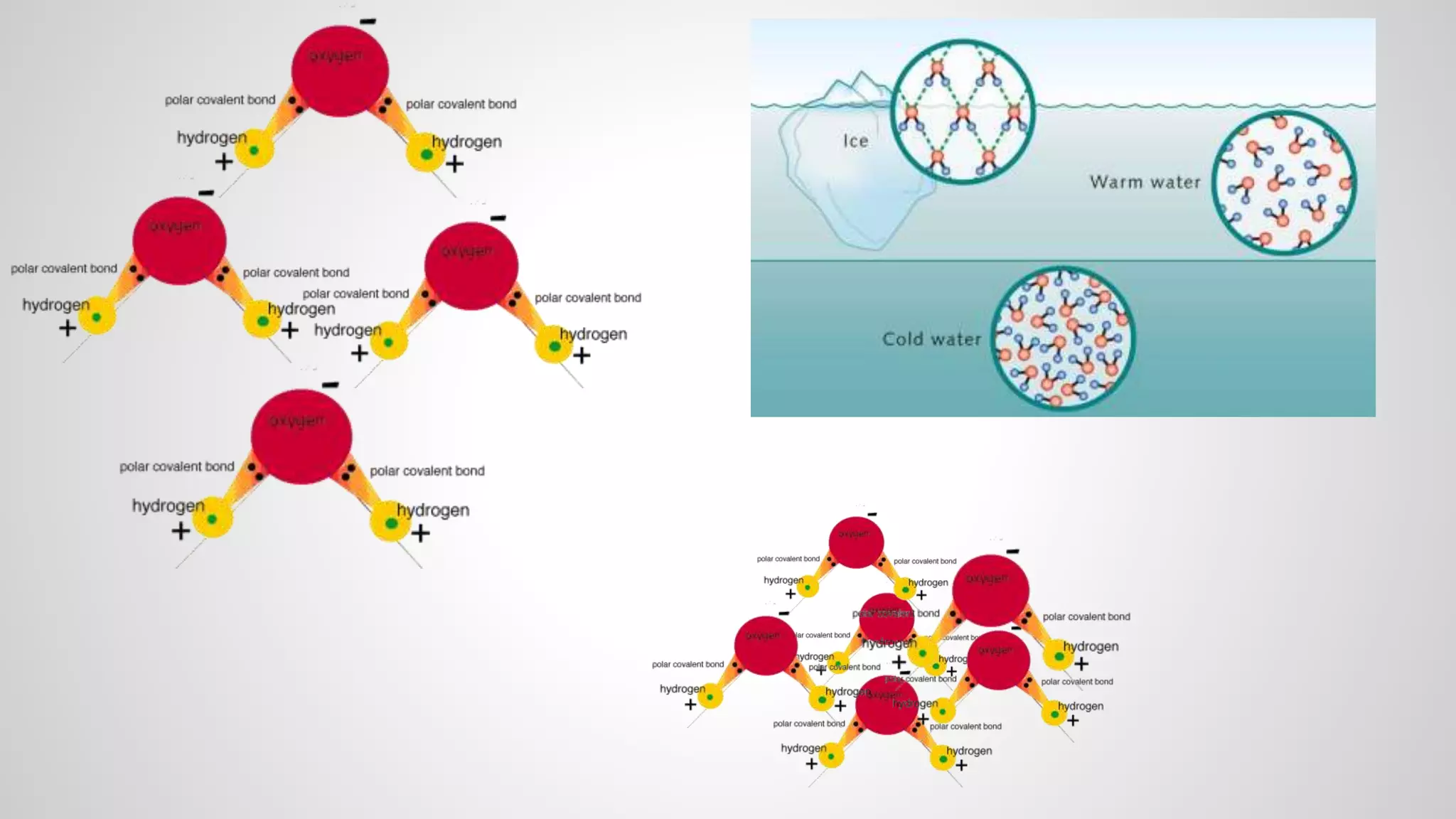
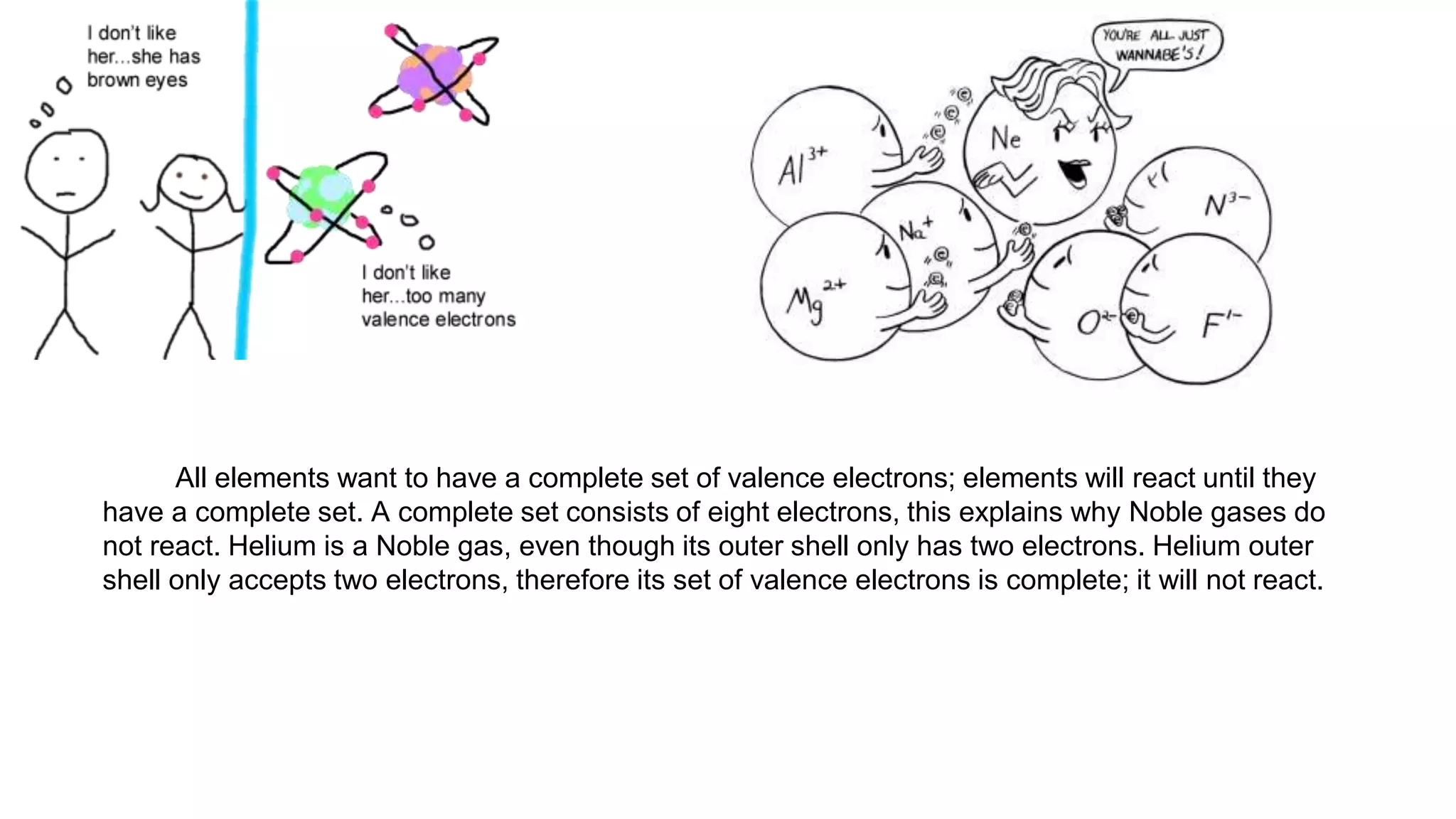
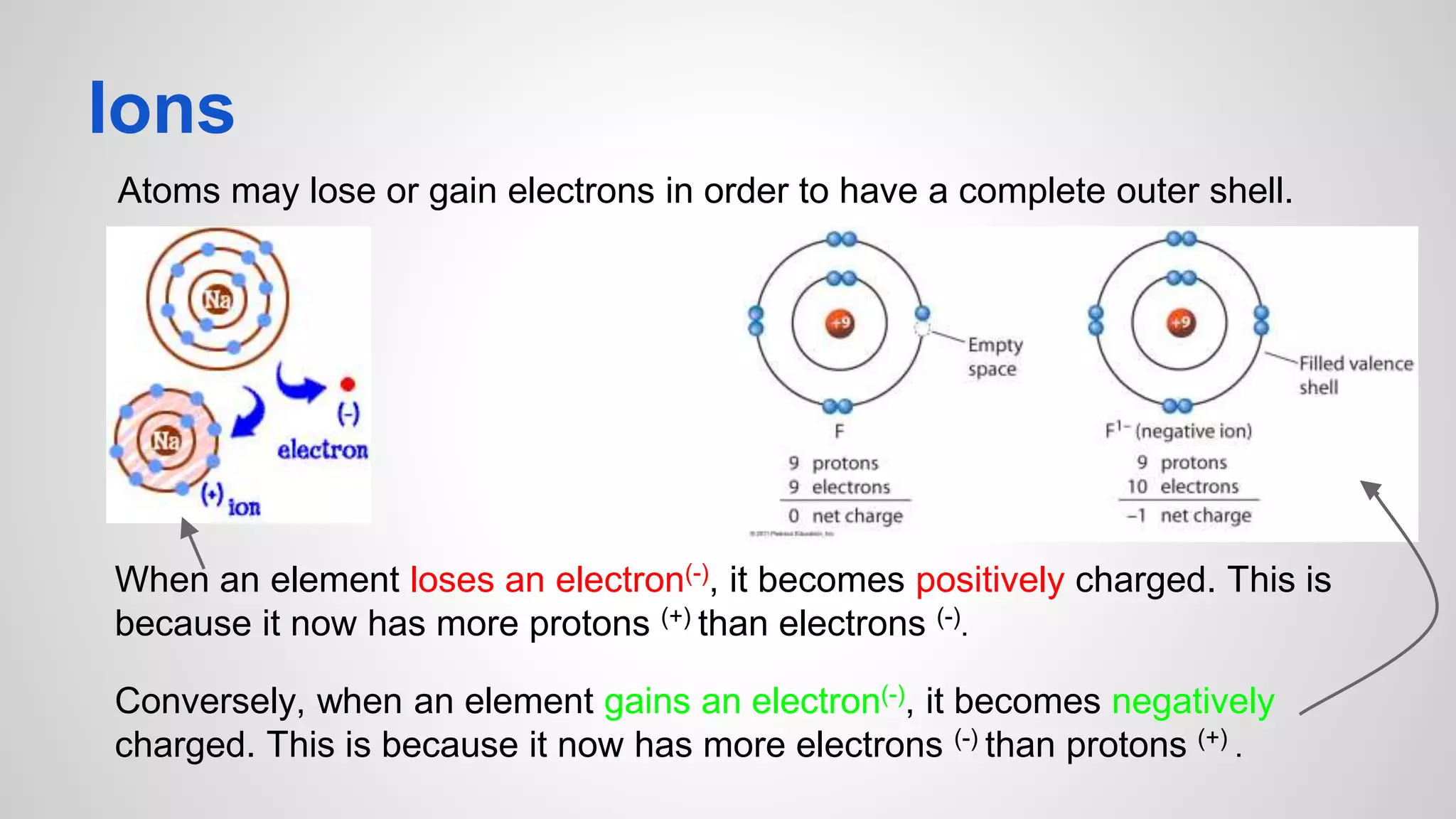
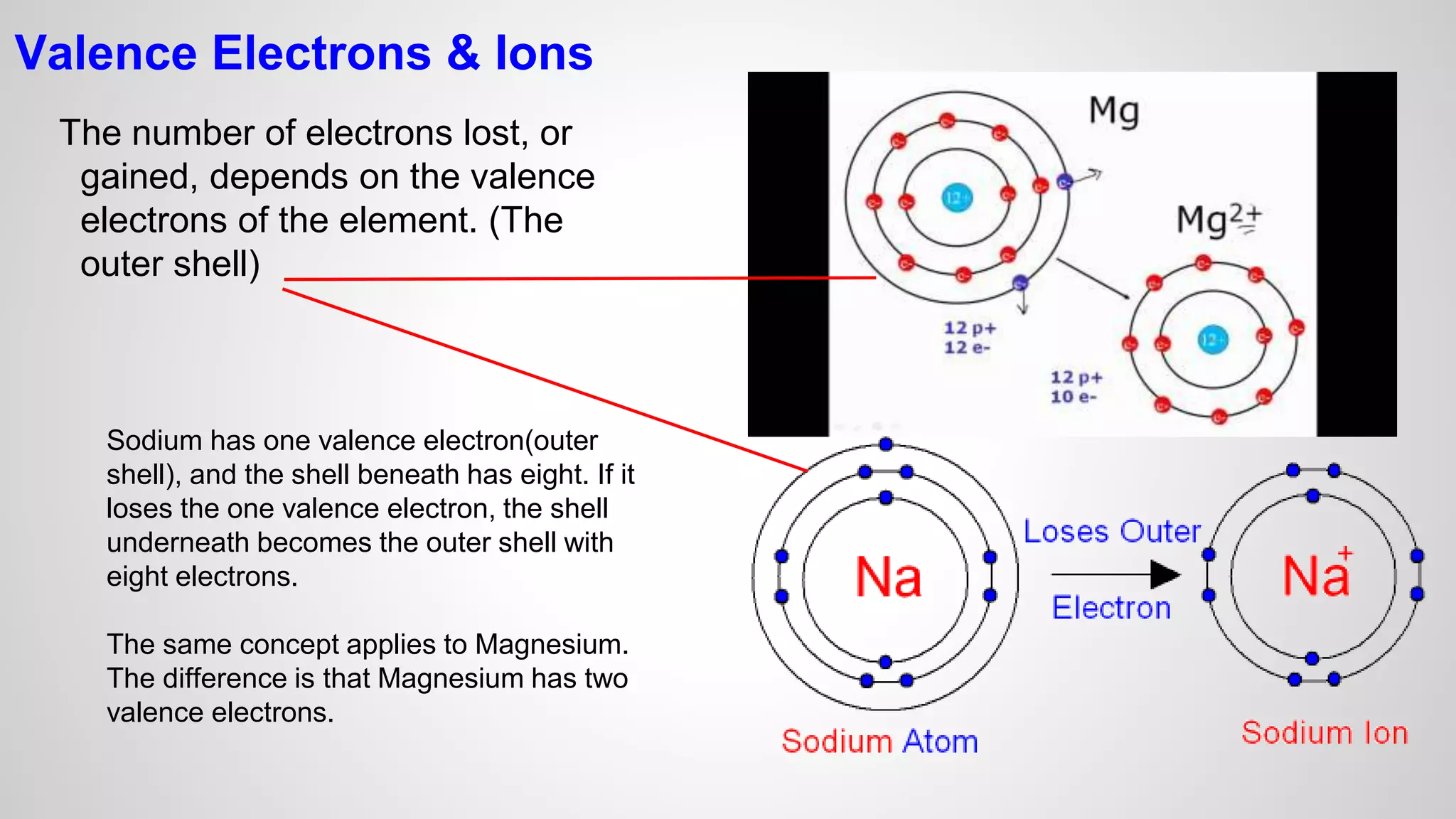
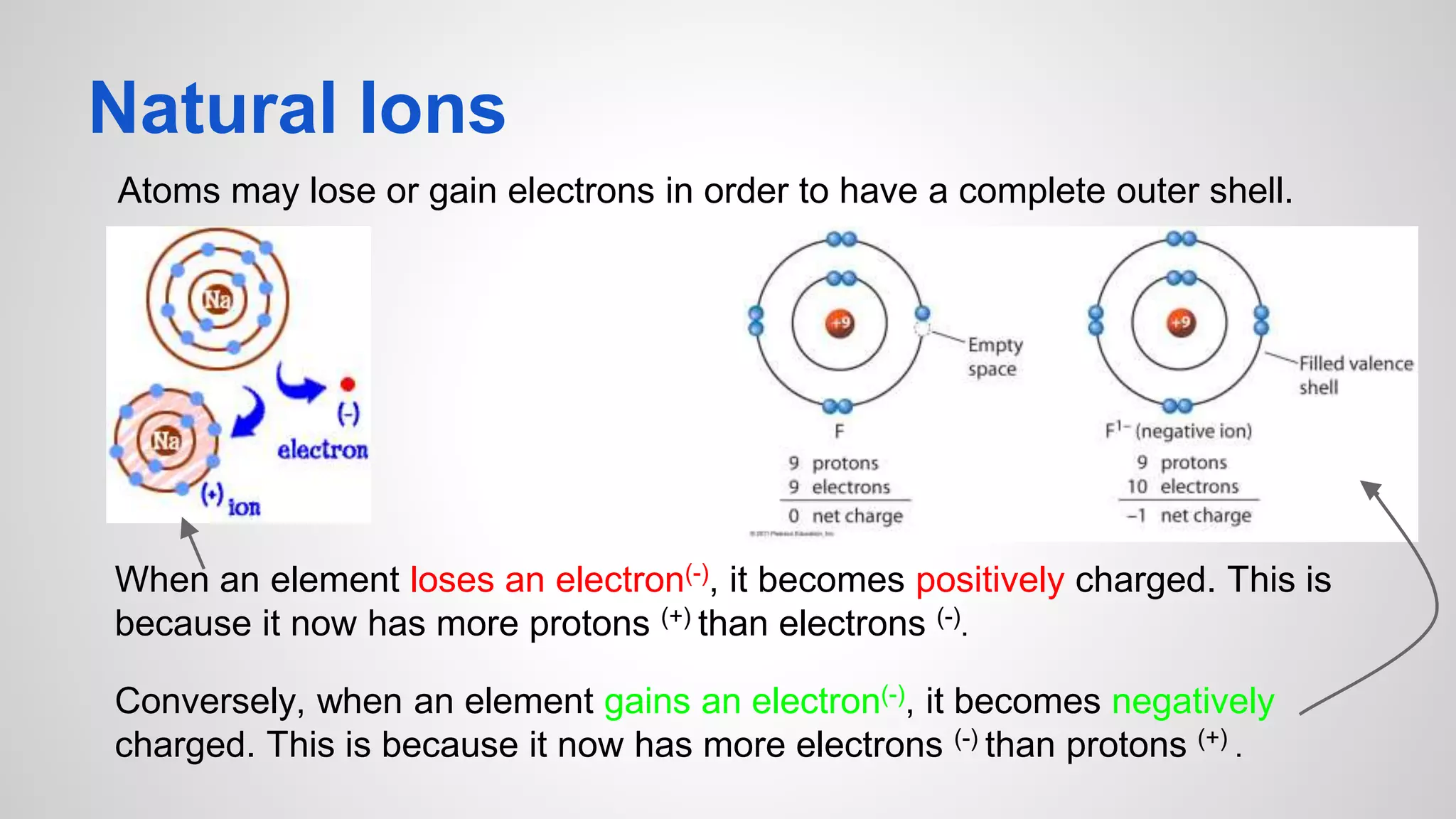
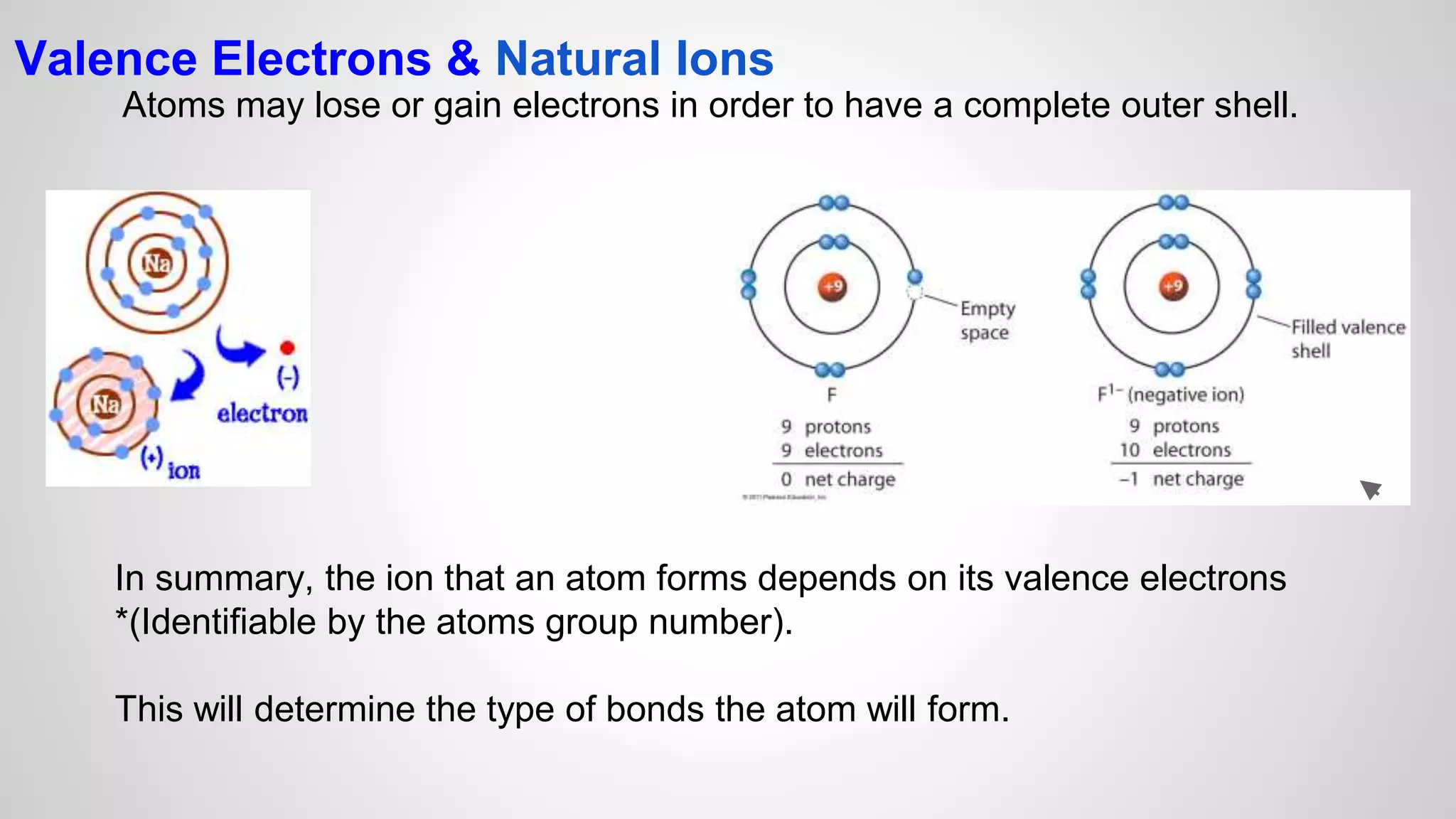
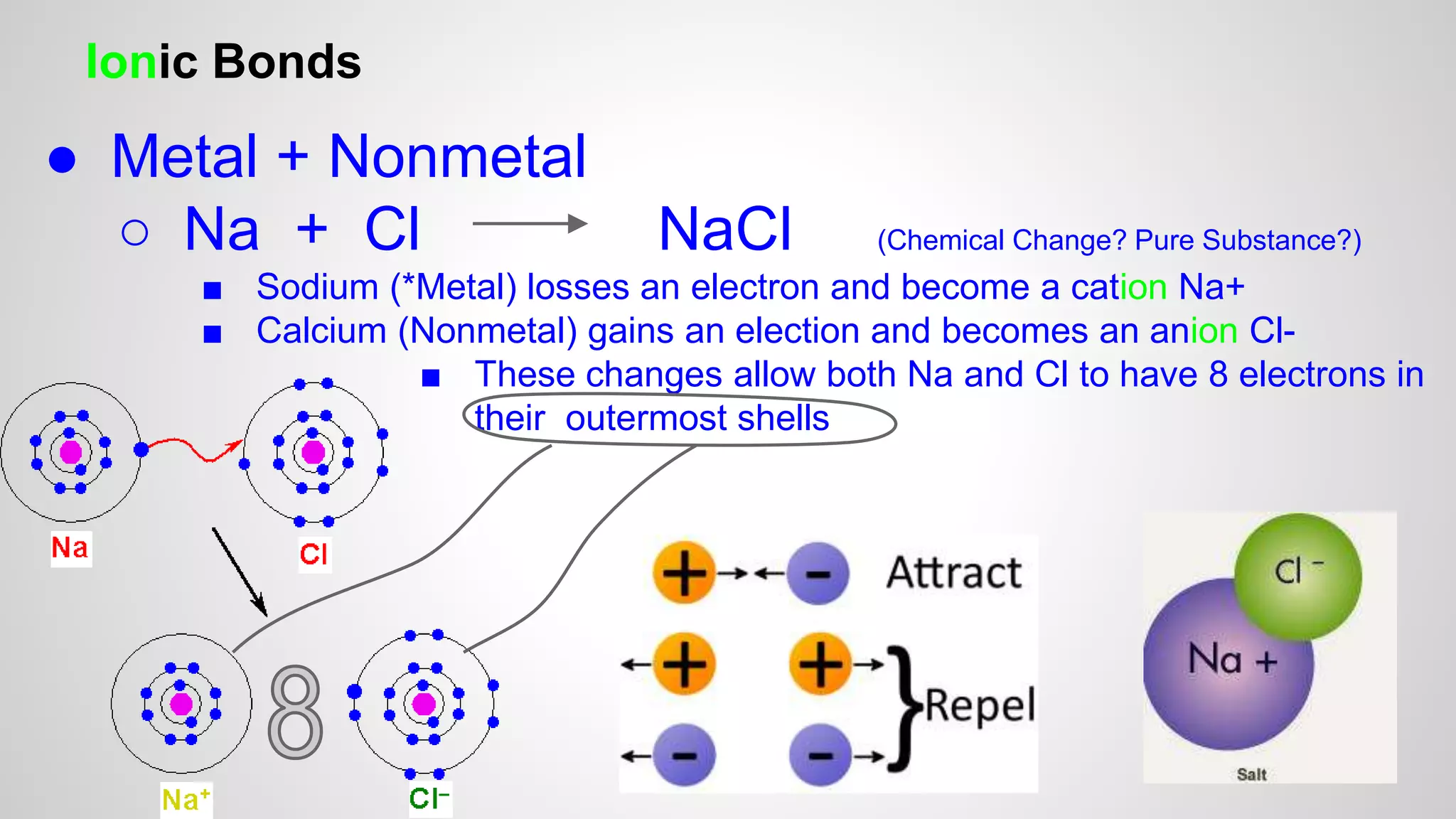
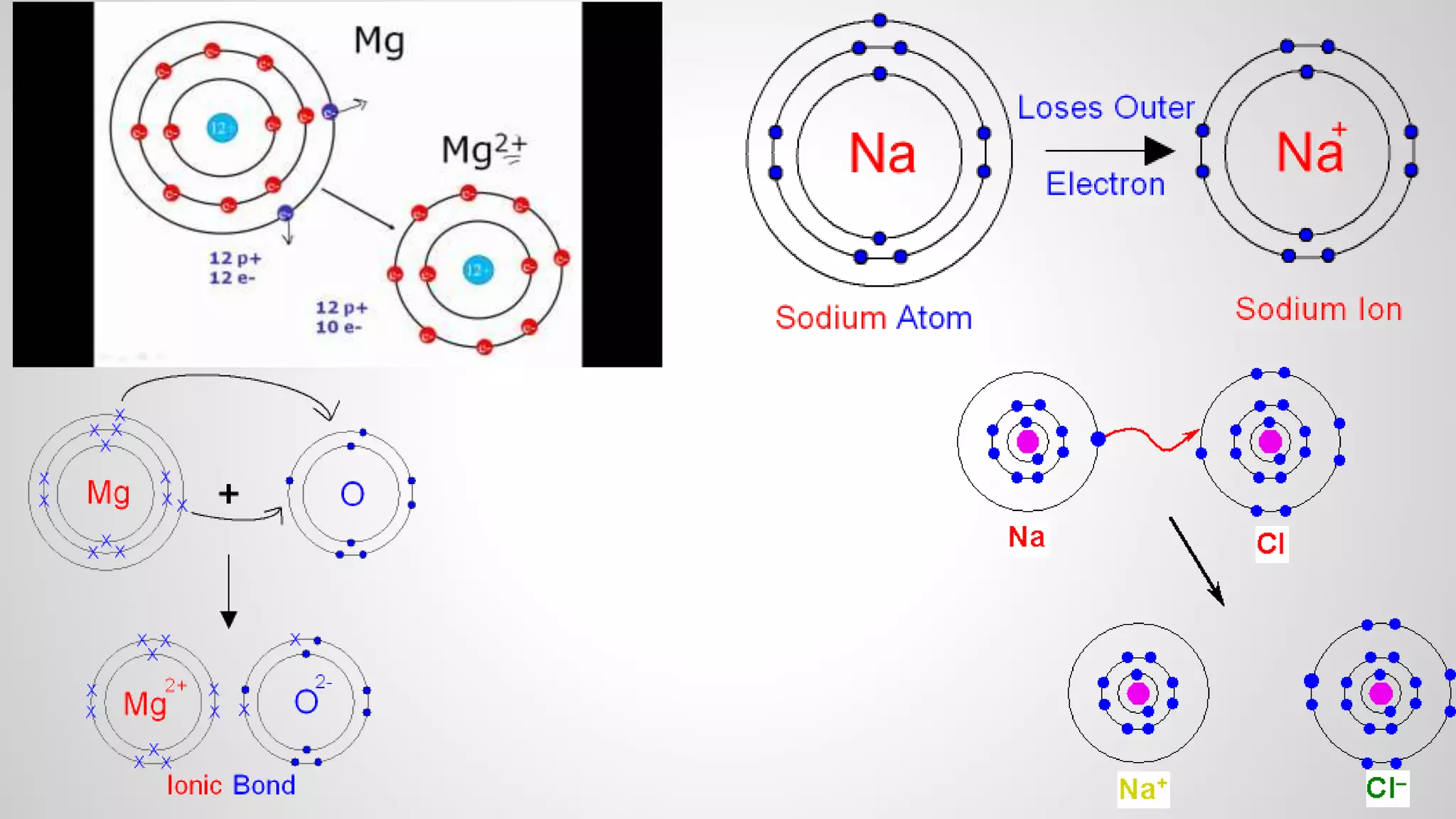
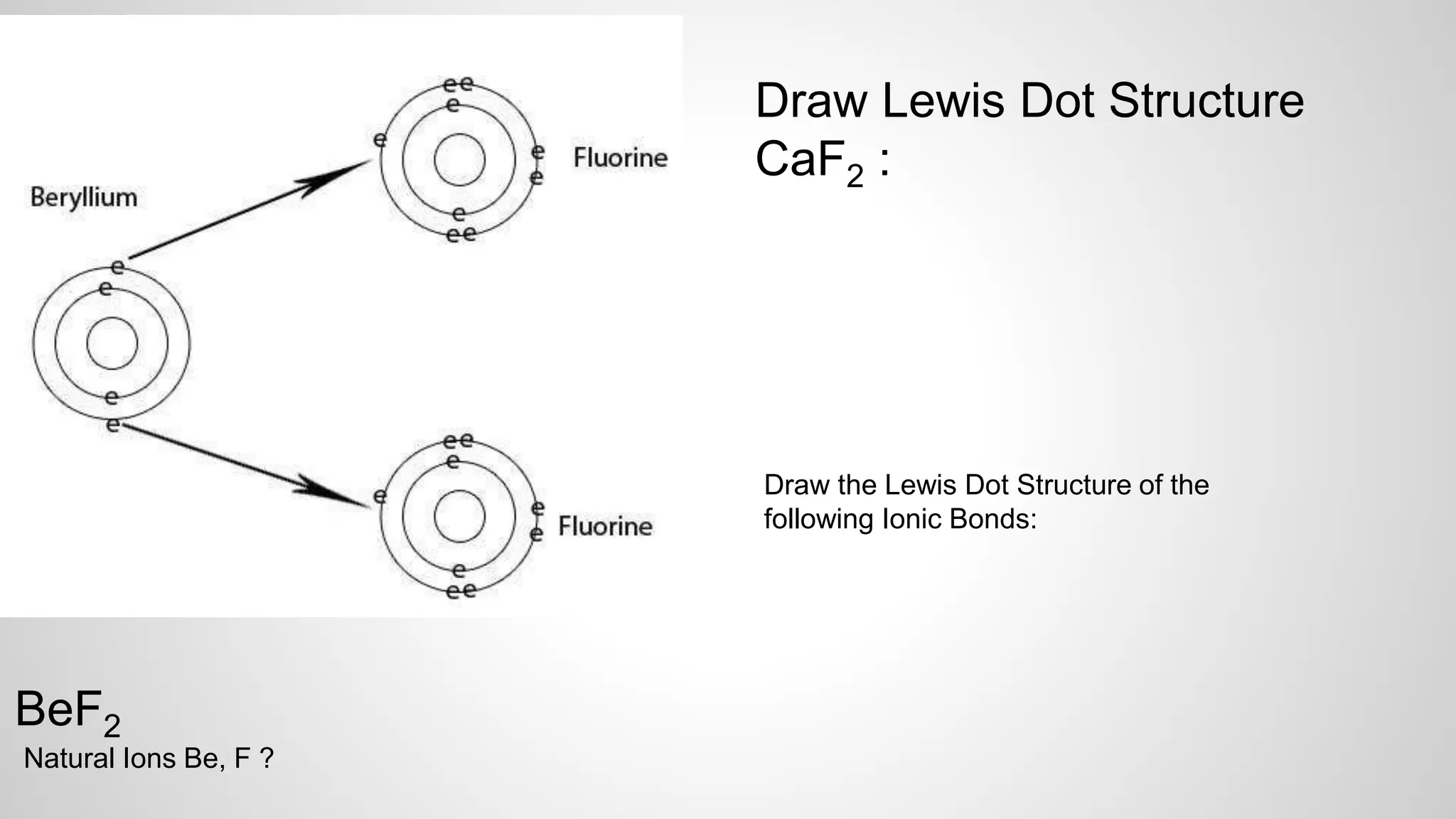
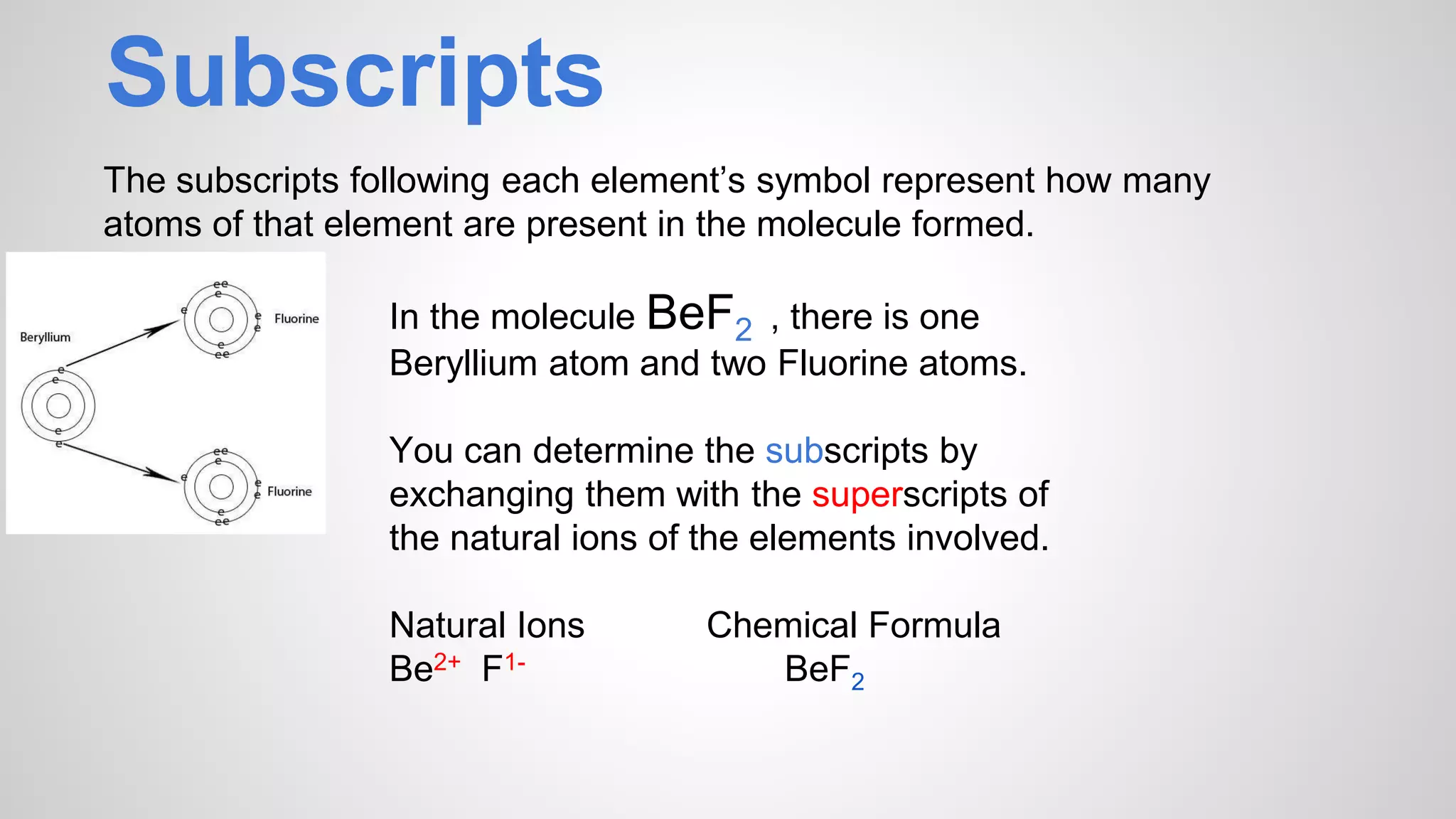
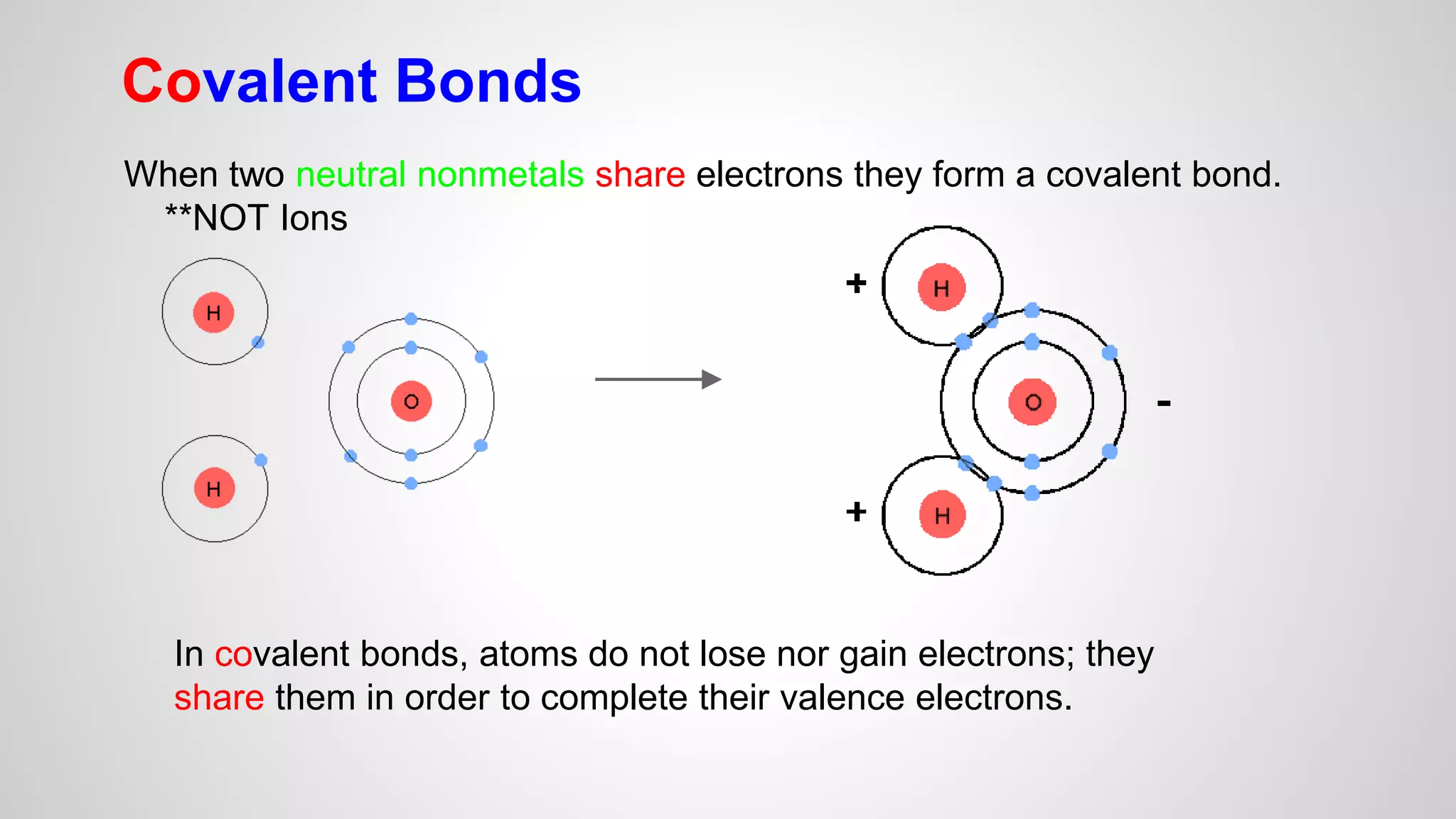
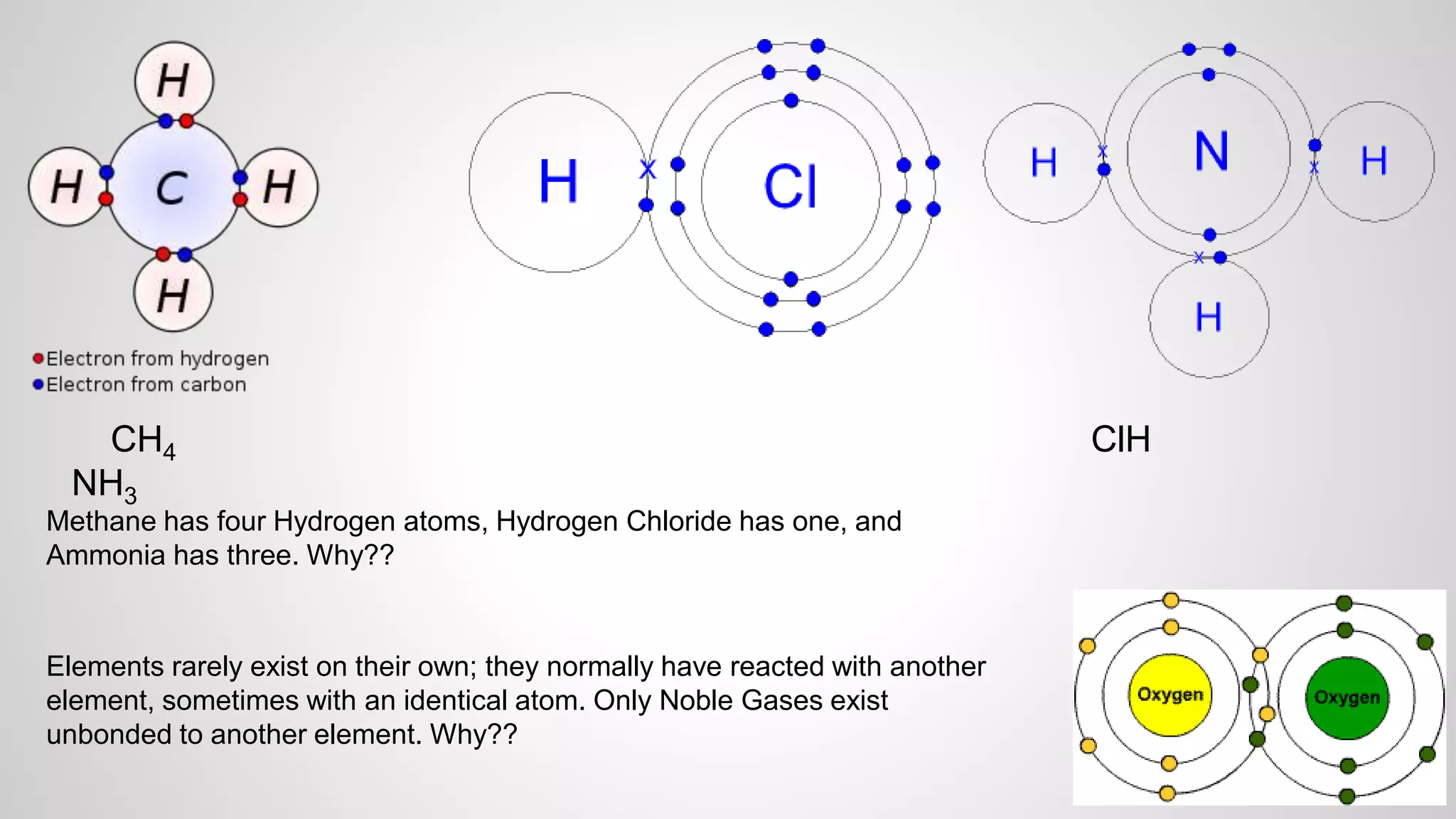
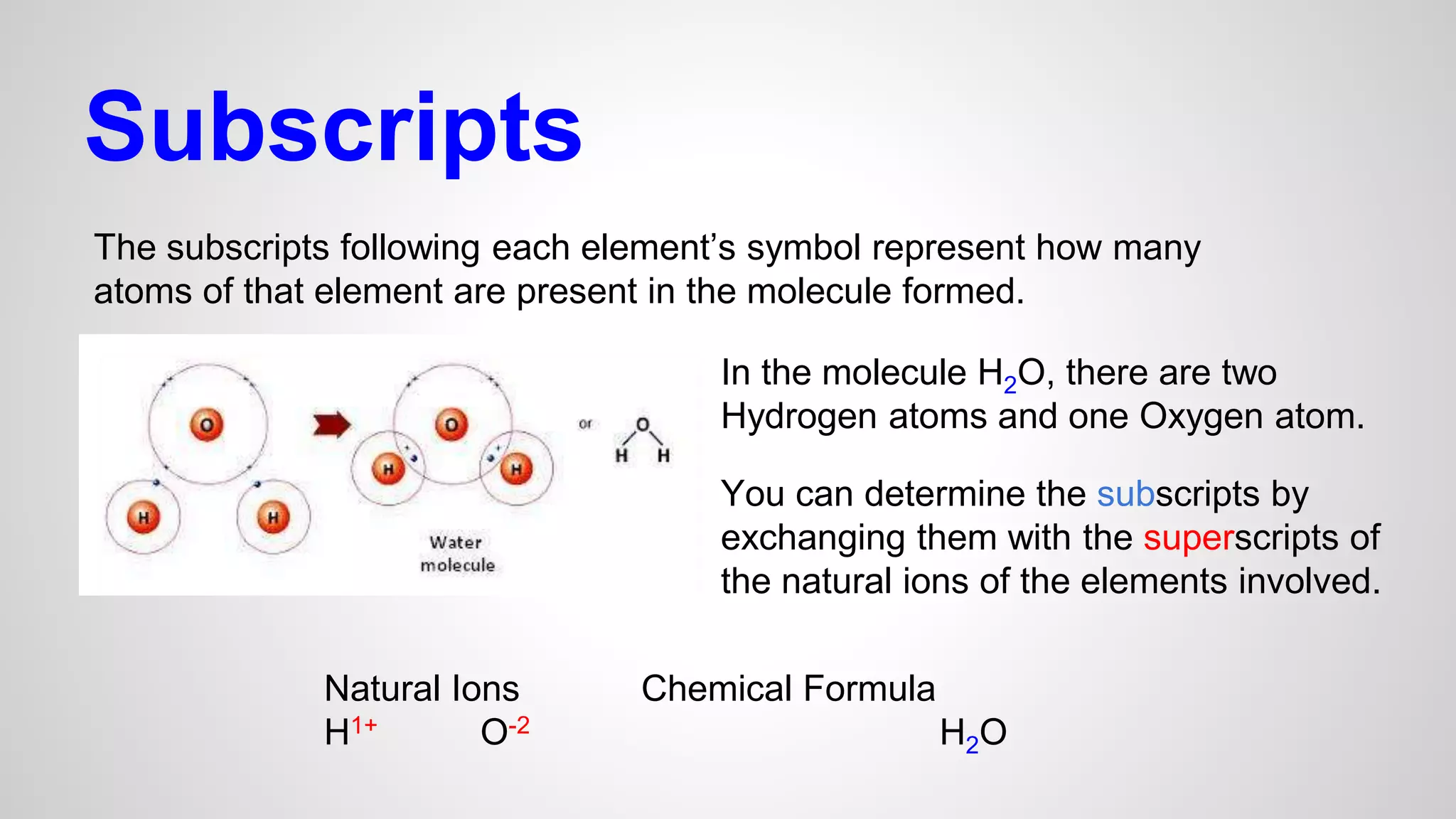
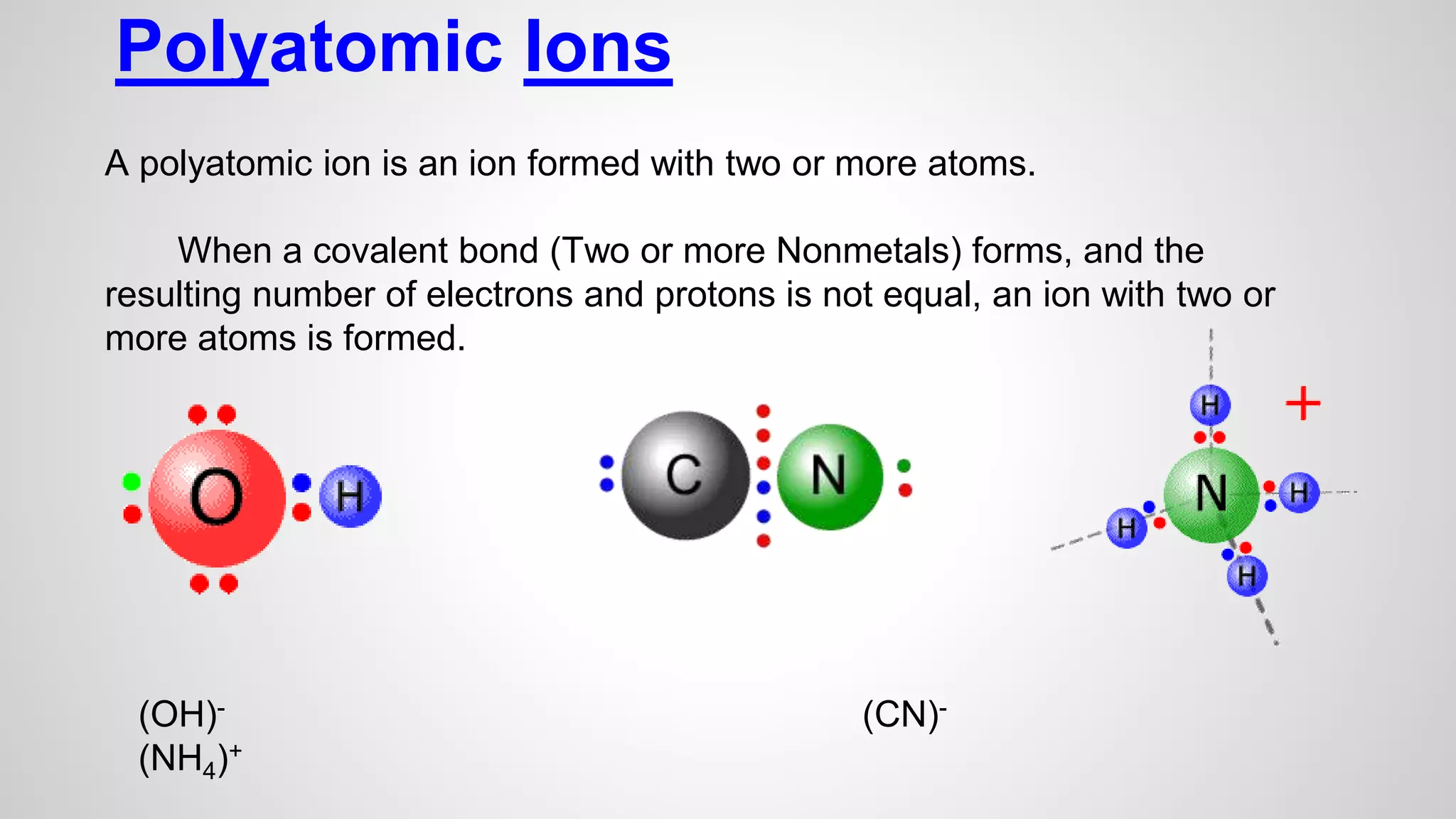
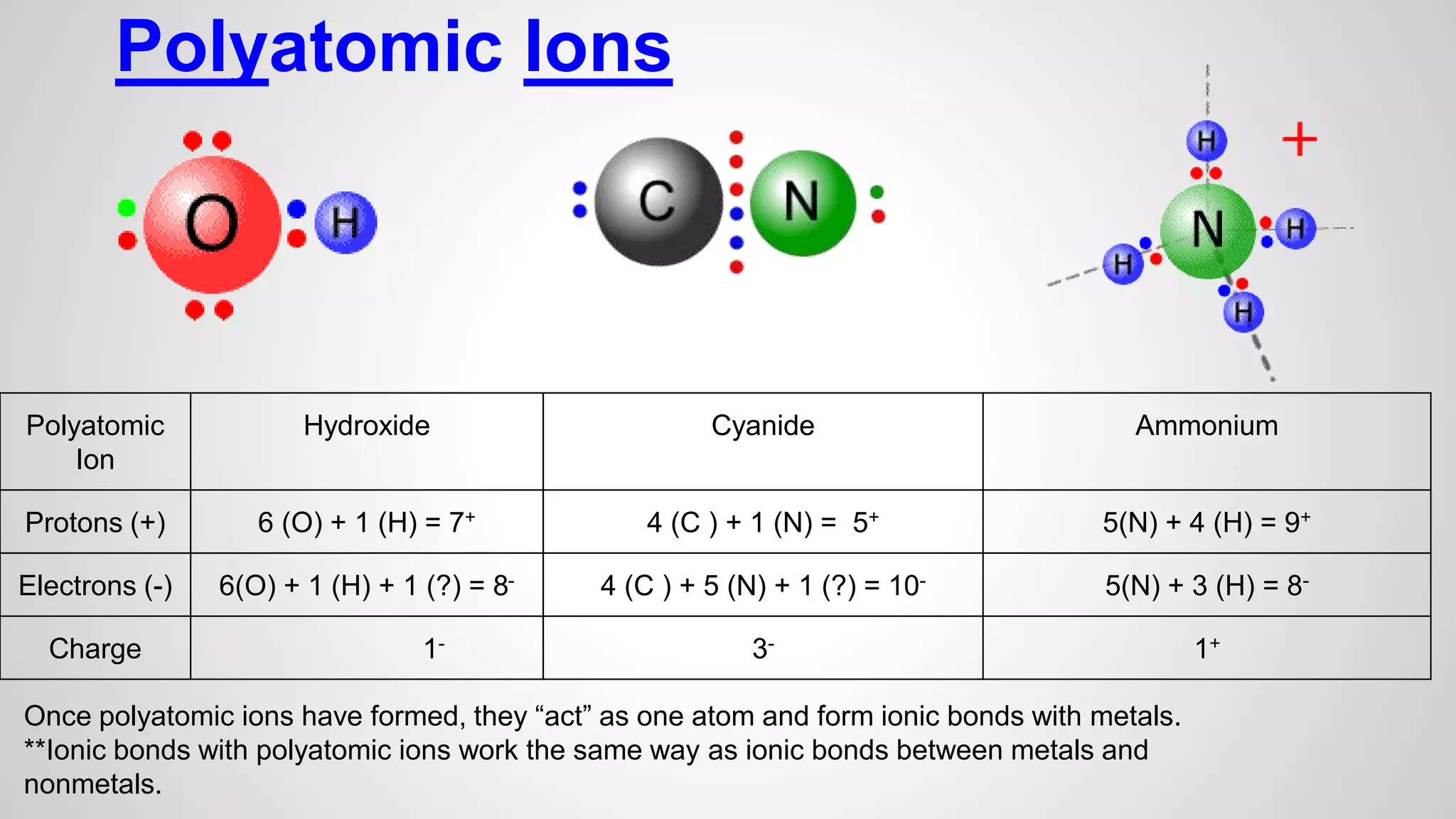
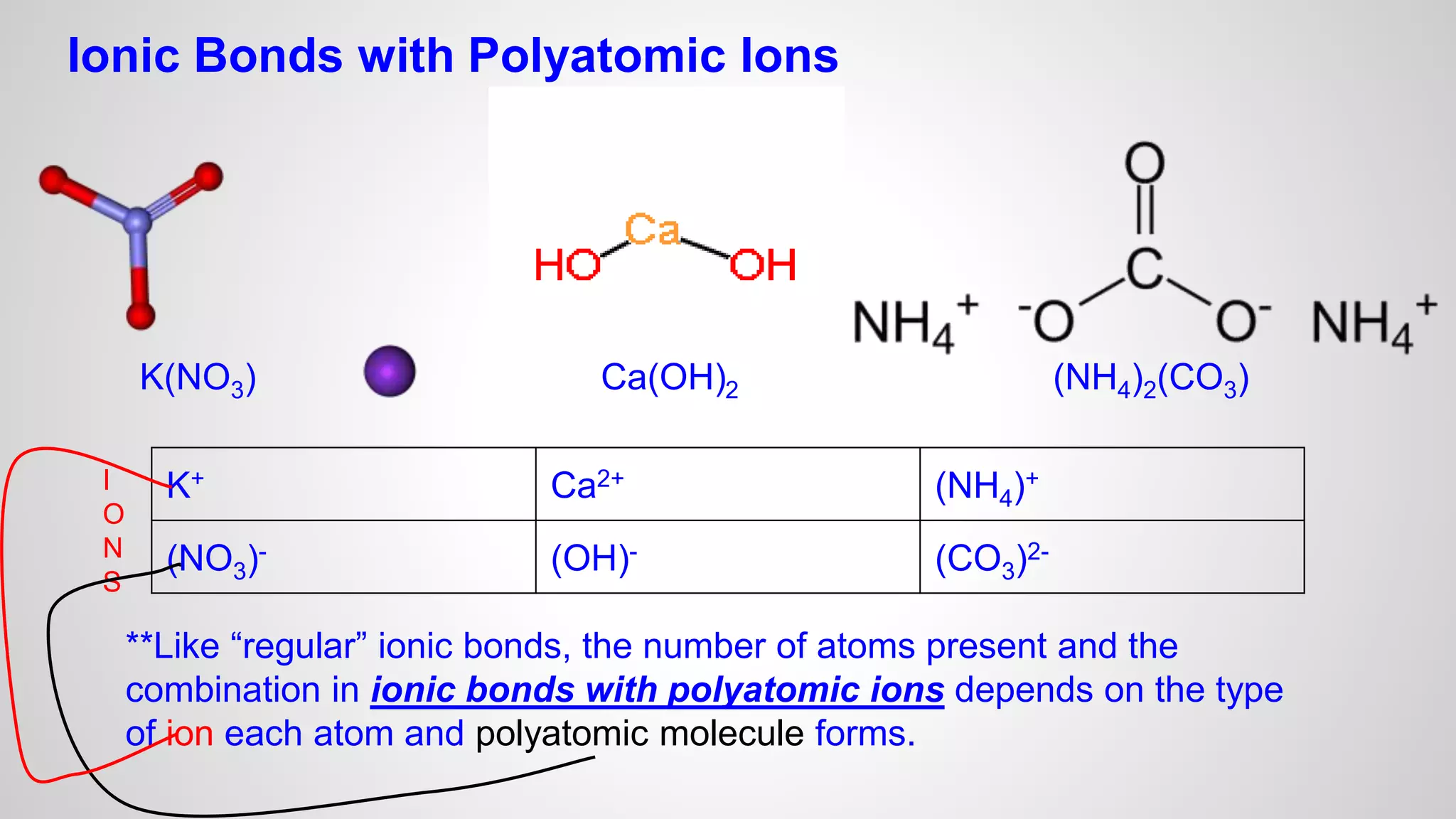
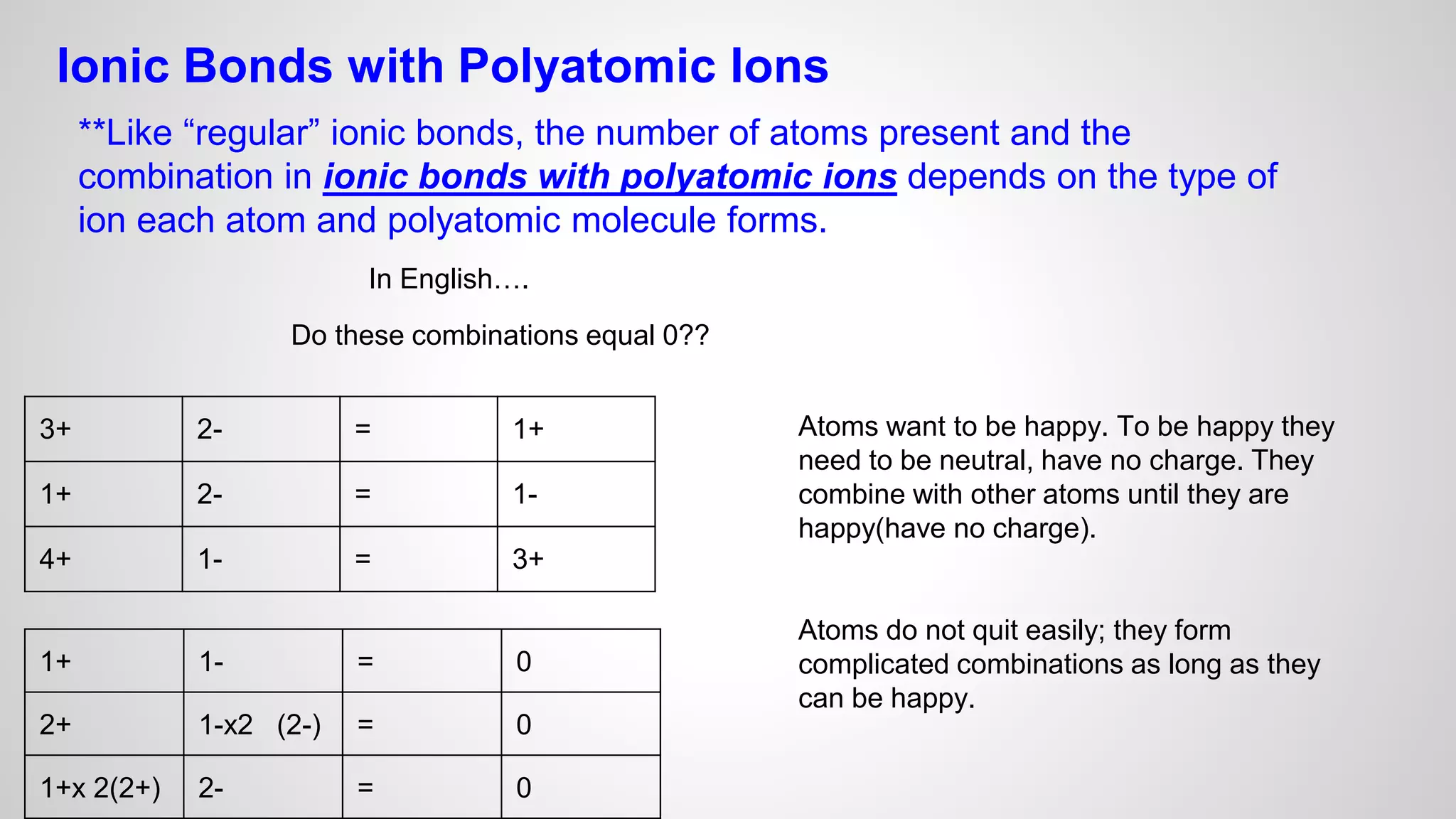
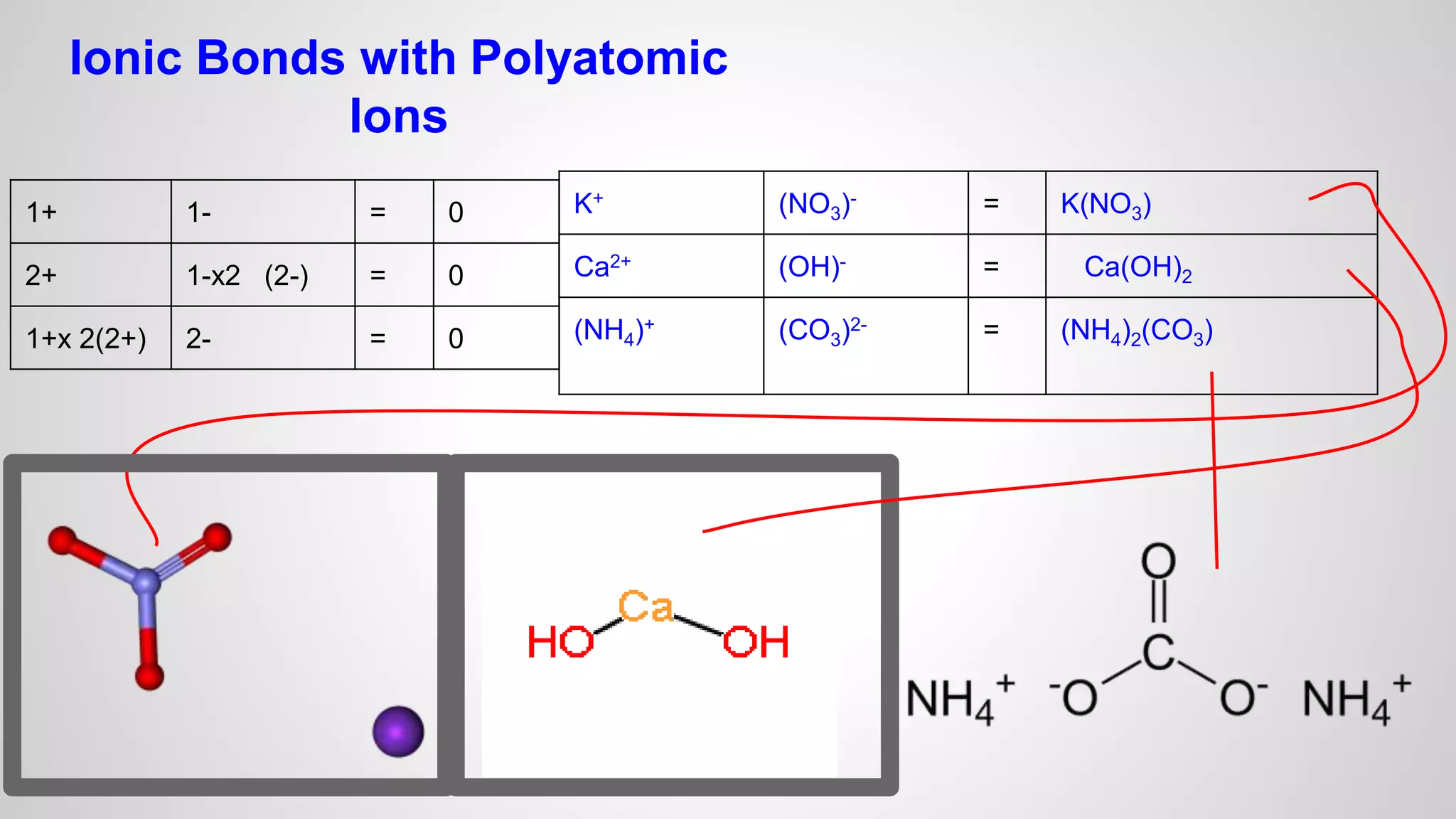
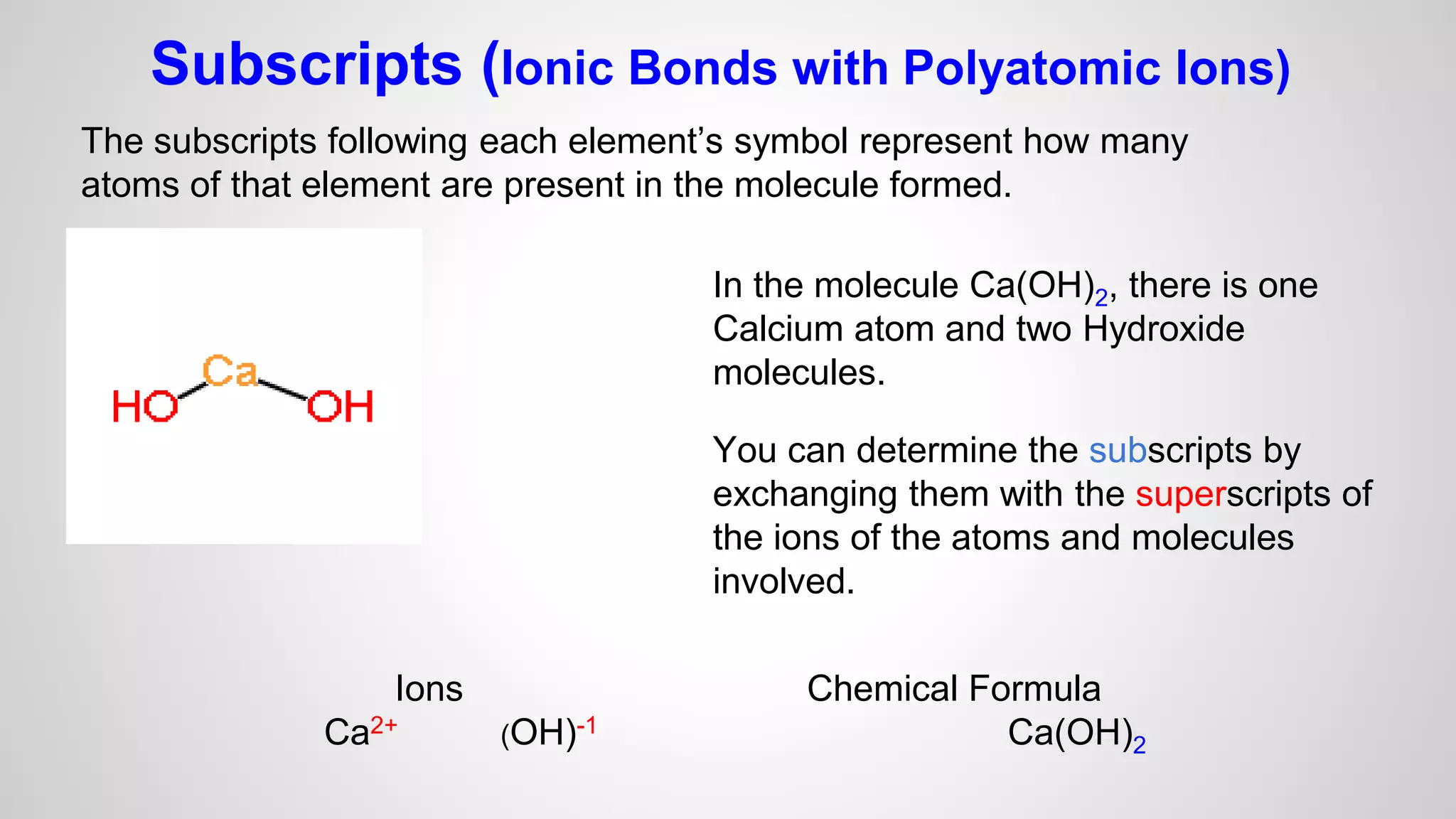
This document defines key terms related to chemical bonds and discusses how atoms bond to achieve stable electron configurations. It explains that atoms form ions by gaining or losing electrons to achieve a full outer electron shell like noble gases. Metals typically lose electrons to form positive ions while nonmetals gain electrons to form negative ions. These oppositely charged ions then form ionic bonds. The document also describes how nonmetal atoms can form covalent bonds by sharing electrons to achieve full outer shells. Polyatomic ions, which are groups of bonded atoms that act as a single unit, are presented and examples are given of how they form ionic bonds with metals through electron transfer.