Embed presentation
Downloaded 171 times

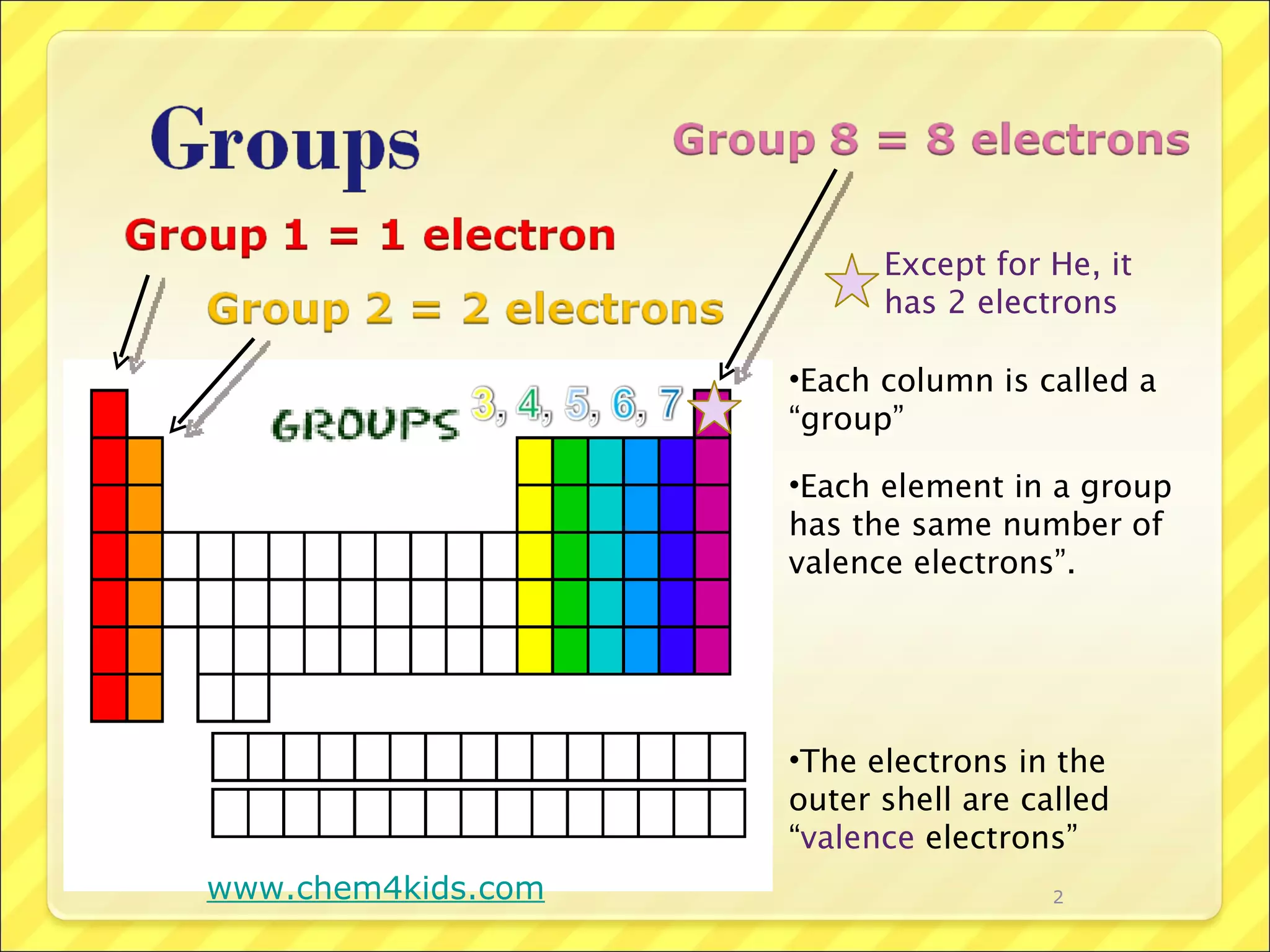
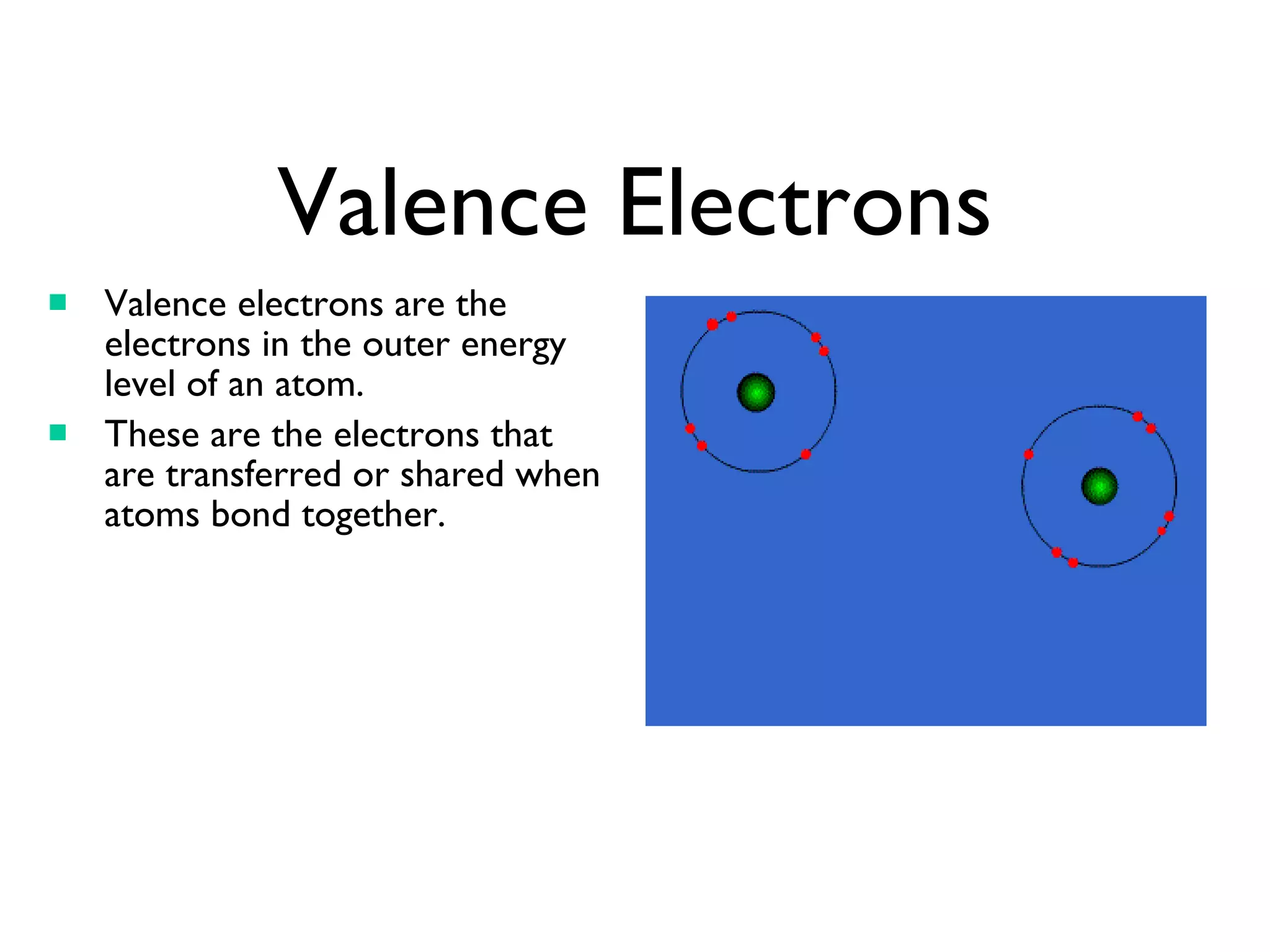



















Each row of the periodic table is called a period. Elements in the same period have the same number of electron shells. Each column is called a group. Elements in the same group have the same number of valence electrons, except for helium which has two electrons. Valence electrons are the outermost electrons of an atom and are involved in bonding. Reactive elements bond easily to gain or lose valence electrons to achieve a full outer electron shell of eight electrons.





















