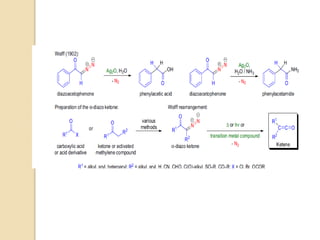
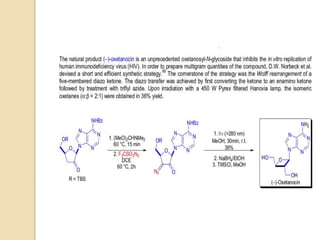
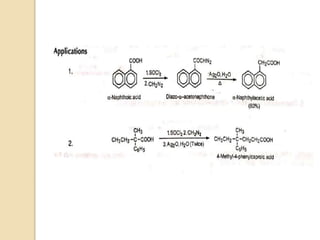
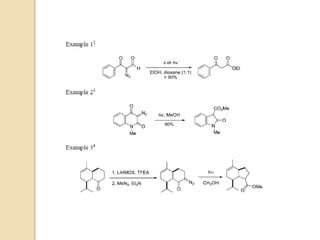
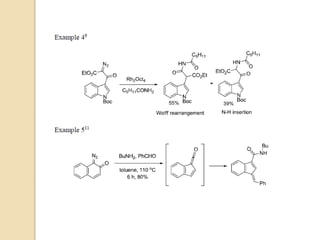
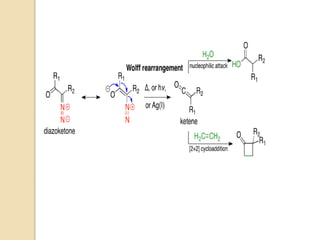
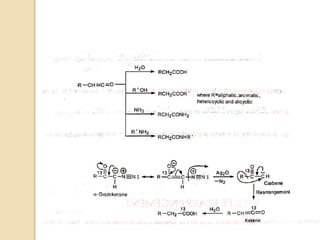
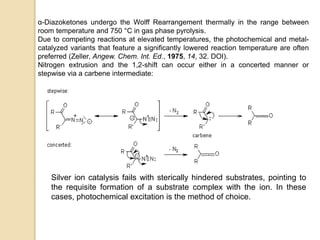
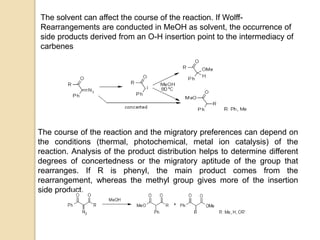
The document summarizes the Wolff rearrangement reaction, which was discovered by German chemist Ludwig Wolff in 1902. The Wolff rearrangement involves the conversion of an α-diazocarbonyl compound into a ketene through loss of dinitrogen with an accompanying 1,2-rearrangement. This yields a ketene intermediate that can undergo nucleophilic attack or [2+2] cycloaddition reactions. The reaction proceeds through either a concerted or stepwise carbene-mediated mechanism. The Wolff rearrangement has synthetic utility but also limitations due to the reactivity of α-diazocarbonyl compounds.


![The Wolff rearrangement is a reaction in organic chemistry in which an α-
diazocarbonyl compound is converted into a ketene by loss of dinitrogen
with accompanying 1,2-rearrangement.
The Wolff rearrangement yields a ketene as an intermediate product, which
can undergo nucleophilic attack with weakly acidic nucleophiles such
as water, alcohols, and amines, to generate carboxylic acid derivatives or
undergo [2+2] cycloaddition reactions to form four-membered rings.
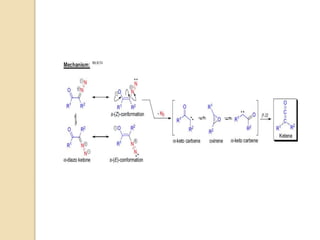
The mechanism of the Wolff rearrangement has been the subject of debate
since its first use. No single mechanism sufficiently describes the reaction,
and there are often competing concerted and carbene-mediated pathways;
for simplicity, only the textbook, concerted mechanism is shown below.
The reaction was discovered by Ludwig Wolff in 1902.
The Wolff rearrangement has great synthetic utility due to the accessibility
of α-diazocarbonyl compounds, variety of reactions from the ketene
intermediate, and stereochemical retention of the migrating
group. However, the Wolff rearrangement has limitations due to the highly
reactive nature of α-diazocarbonyl compounds, which can undergo a variety
of competing reaction](https://image.slidesharecdn.com/wolff-210521165018/85/Wolff-3-320.jpg)
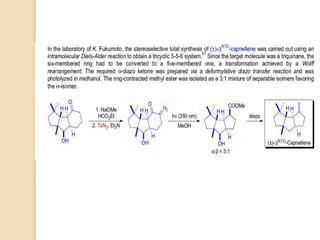
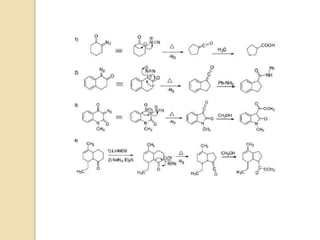
![6) the use of transition metal complexes does not only reduce the required
reaction temperature considerably compared to the thermal process, but also
changes the reactivity of the α-keto carbene intermediate by the formation of
less reactive metal carbene complexes (Rh- and Pd-complexes usually
prevent the Wolff rearrangement from taking place);
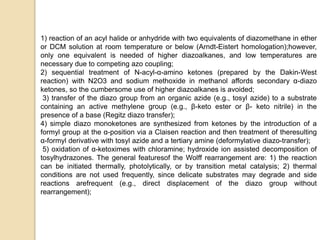
7) freshly prepared silver(I)oxide or silver(I)benzoate are best suited for the
reaction;
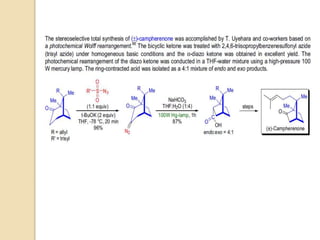
8) photochemical activation is convenient, and it takes place even at low
temperatures, but it can be problematic if the product is photolabile;
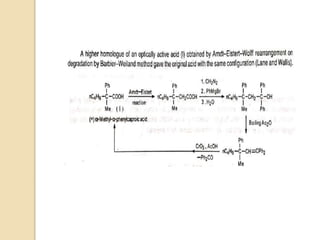
9) if the migrating group has a stereocenter, the stereochemistry remains
unchanged (net retention of configuration) after the migration;
10) theketene products are electrophilic and can react with various
nucleophiles as well as undergo [2+2] cycloaddition reactions with alkenes;
11) cyclic diazo ketones undergo ring-contraction, and the process is well-
suited for the preparation of strained ring systems;
12) α,β-unsaturated diazo ketones undergo the vinylogous Wolff
rearrangementto give skeletally rearranged γ,δ-unsaturated esters (alternative
to Claisen-type rearrangements);1and
13) since α-diazo ketones are very reactive compounds, numerous side
reactions are possible that can be avoided or minimized by the careful choice
of reaction conditions.9](https://image.slidesharecdn.com/wolff-210521165018/85/Wolff-10-320.jpg)