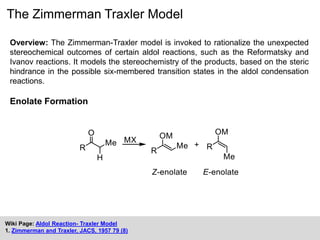
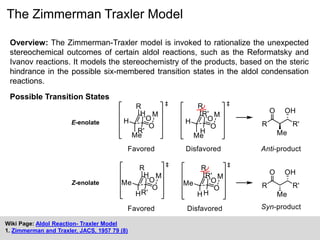
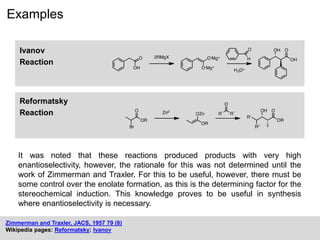
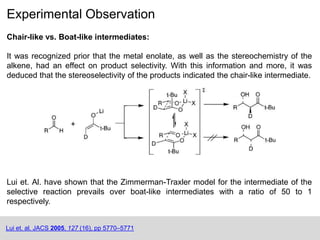
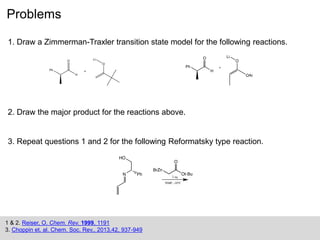
The Zimmerman-Traxler model explains the stereochemical outcomes of certain aldol reactions by modeling six-membered transition states based on steric hindrance. It highlights the necessity of controlling enolate formation for achieving high enantioselectivity in product synthesis. The model has been validated through experimental findings that demonstrate a preference for chair-like over boat-like intermediates.