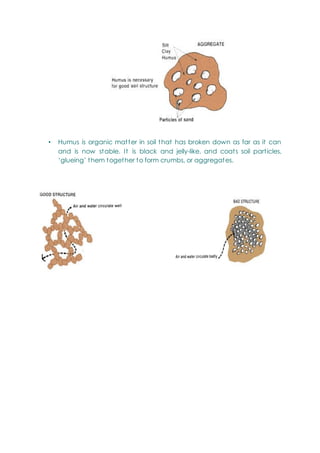
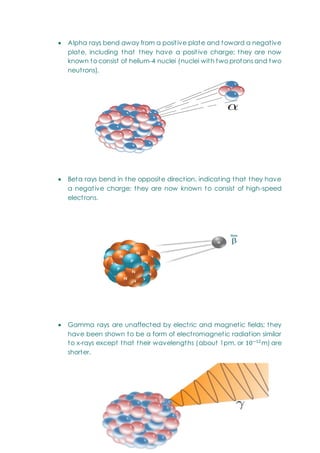
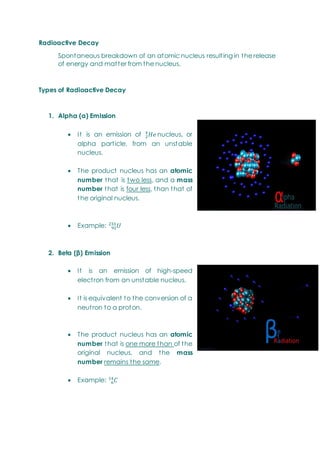
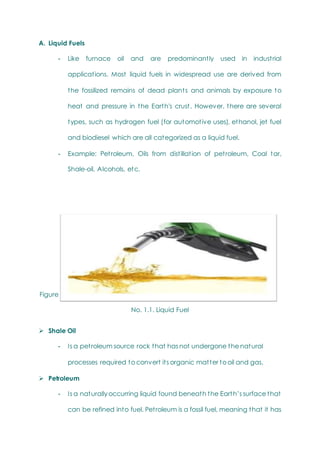
This document provides an overview of chemistry topics relevant to engineering, including:
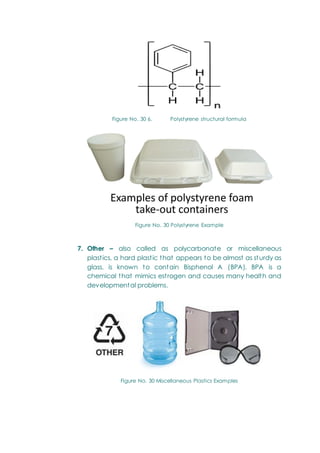
- The chemistry of engineering materials, which classifies materials as metals (ferrous, non-ferrous) and non-metals (synthetic, natural). It describes common materials like steel, aluminum, plastics, ceramics, composites, wood, and rubber.
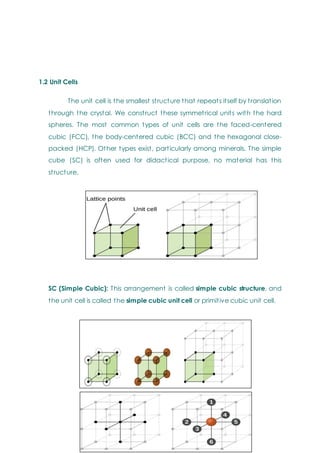
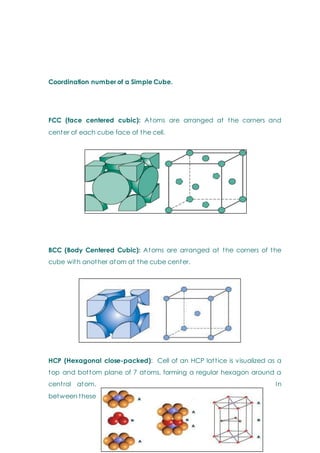
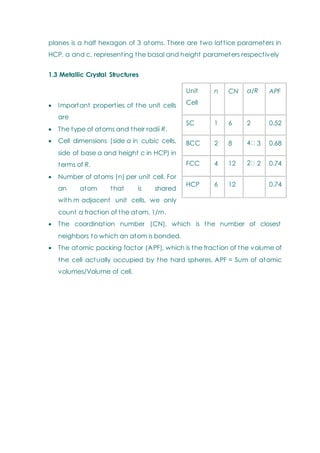
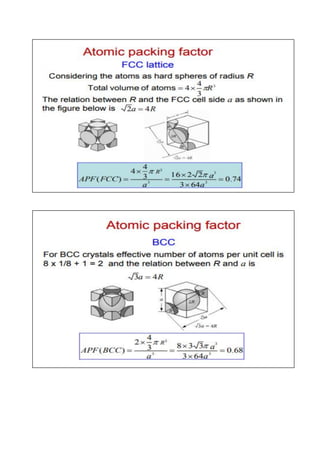
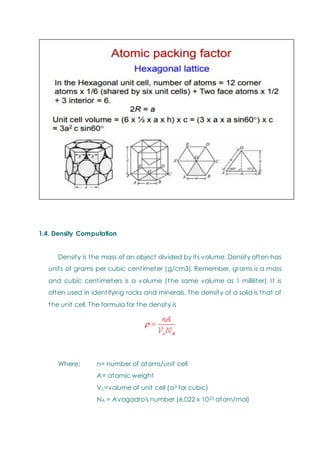
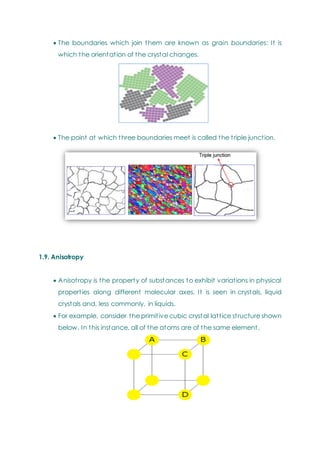
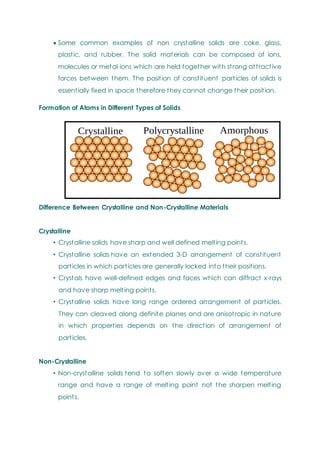
- Basic concepts of crystal structure, including crystalline vs amorphous structure and lattice structures.
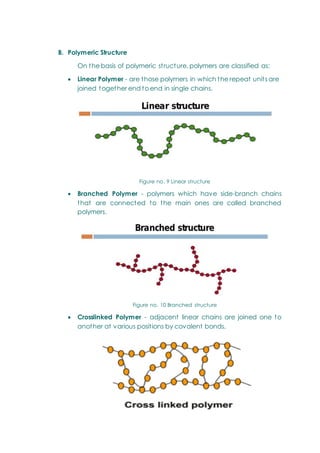
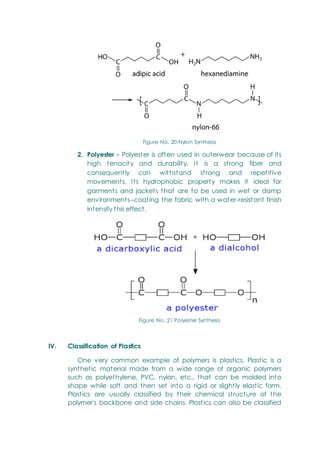
- Polymers, including classifications based on source (natural, semi-synthetic, synthetic), thermal response (thermoplastic, thermosetting), and the polymerization process.
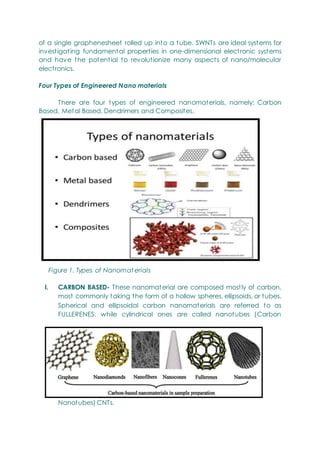
- Engineered nanomaterials, defined as having at least one dimension