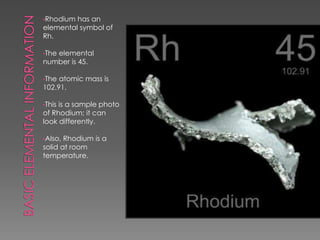
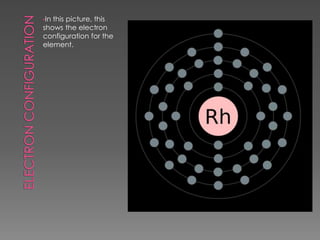
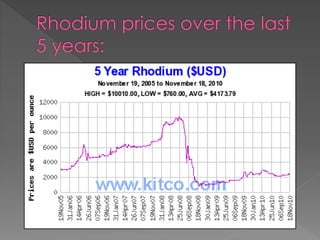
Rhodium is a shiny silver-colored metal that was discovered in England in 1803. It has a high melting point of 1966°C and boiling point of 3727°C. Rhodium is very dense, with a density of 12.4 g/cm3, higher than water and steel. It is commonly found with nickel and copper in South America and Canada. Rhodium is primarily used for plating jewelry and coins and alloying with platinum and palladium for hardness.