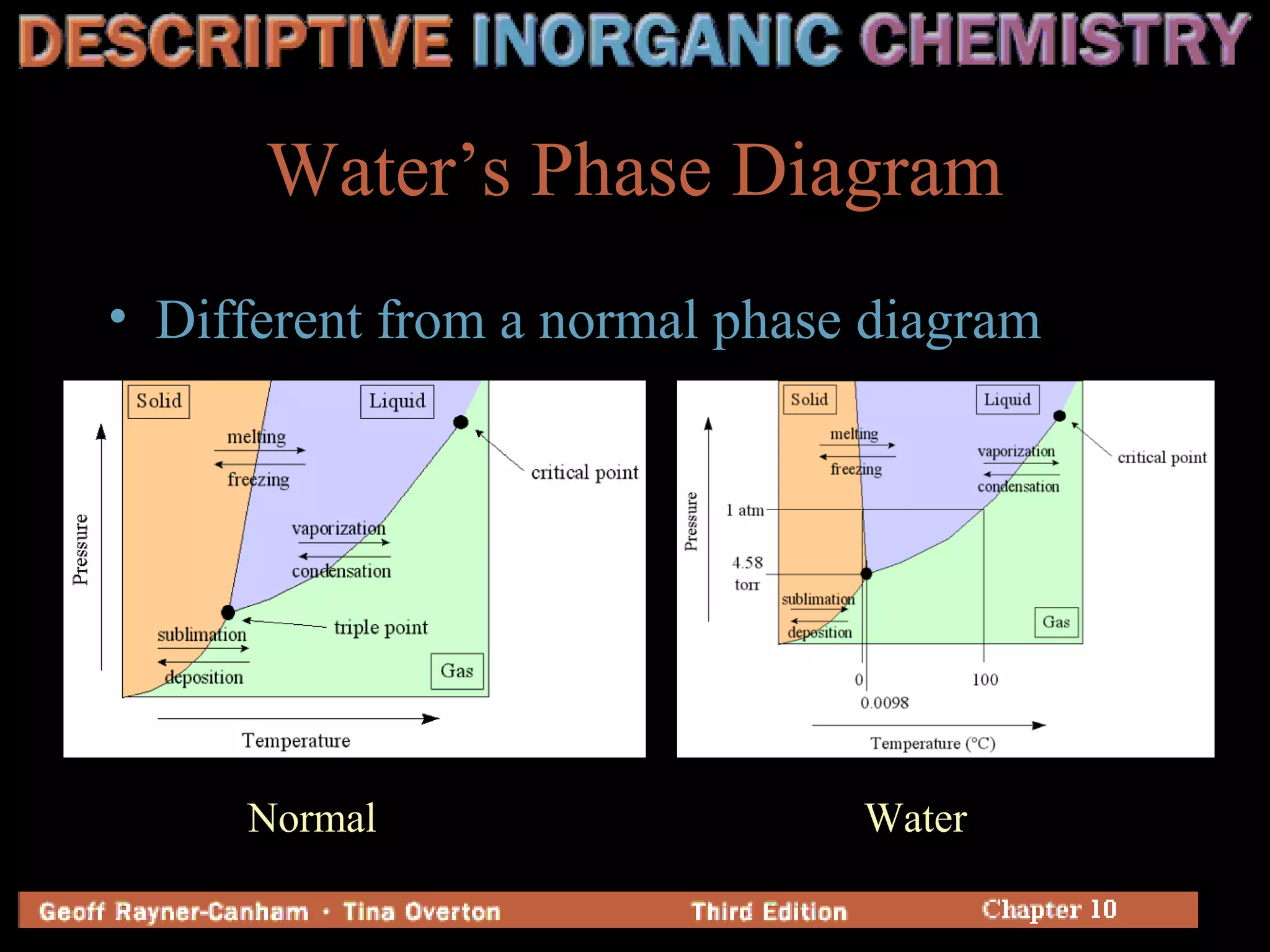
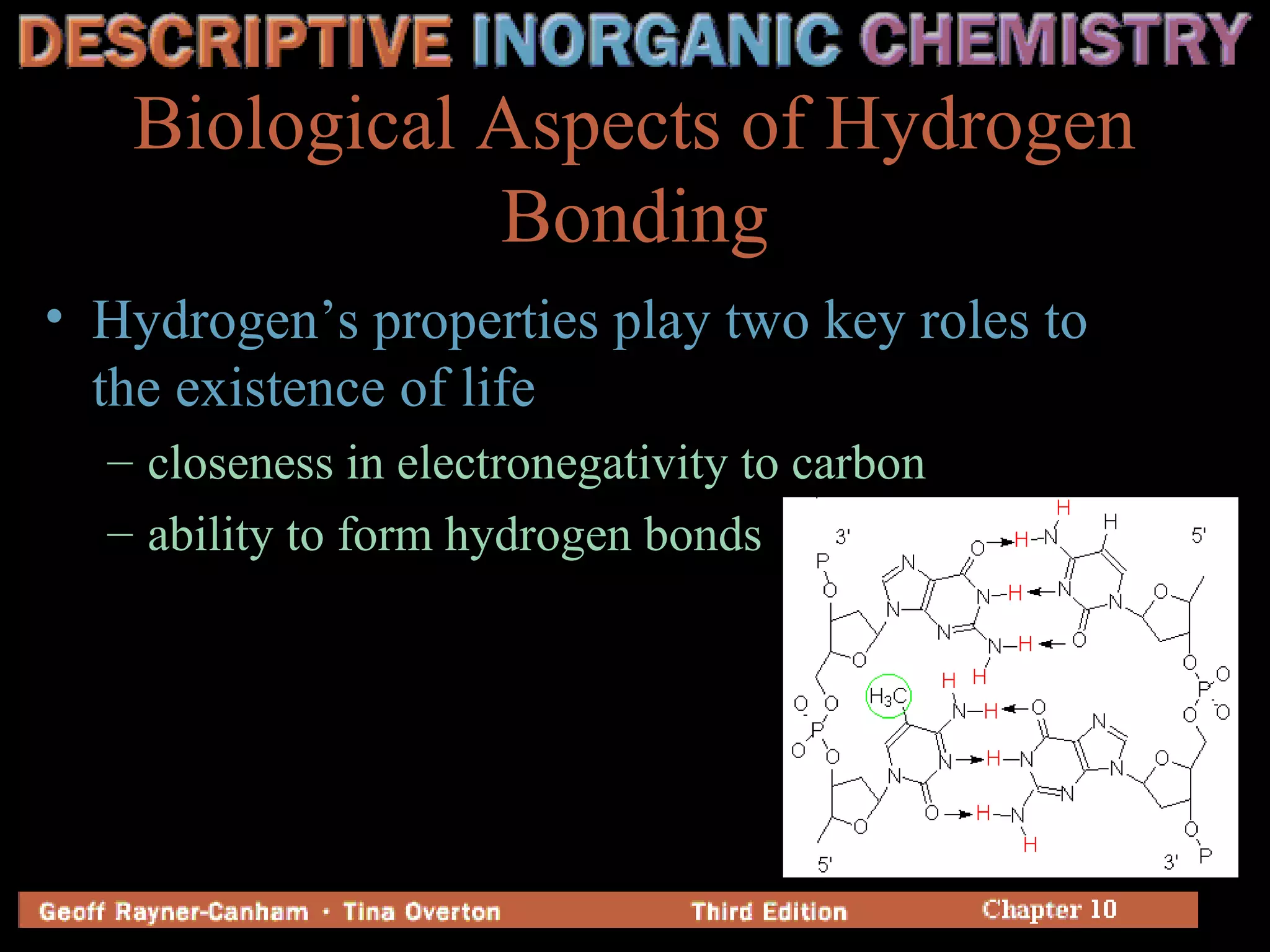
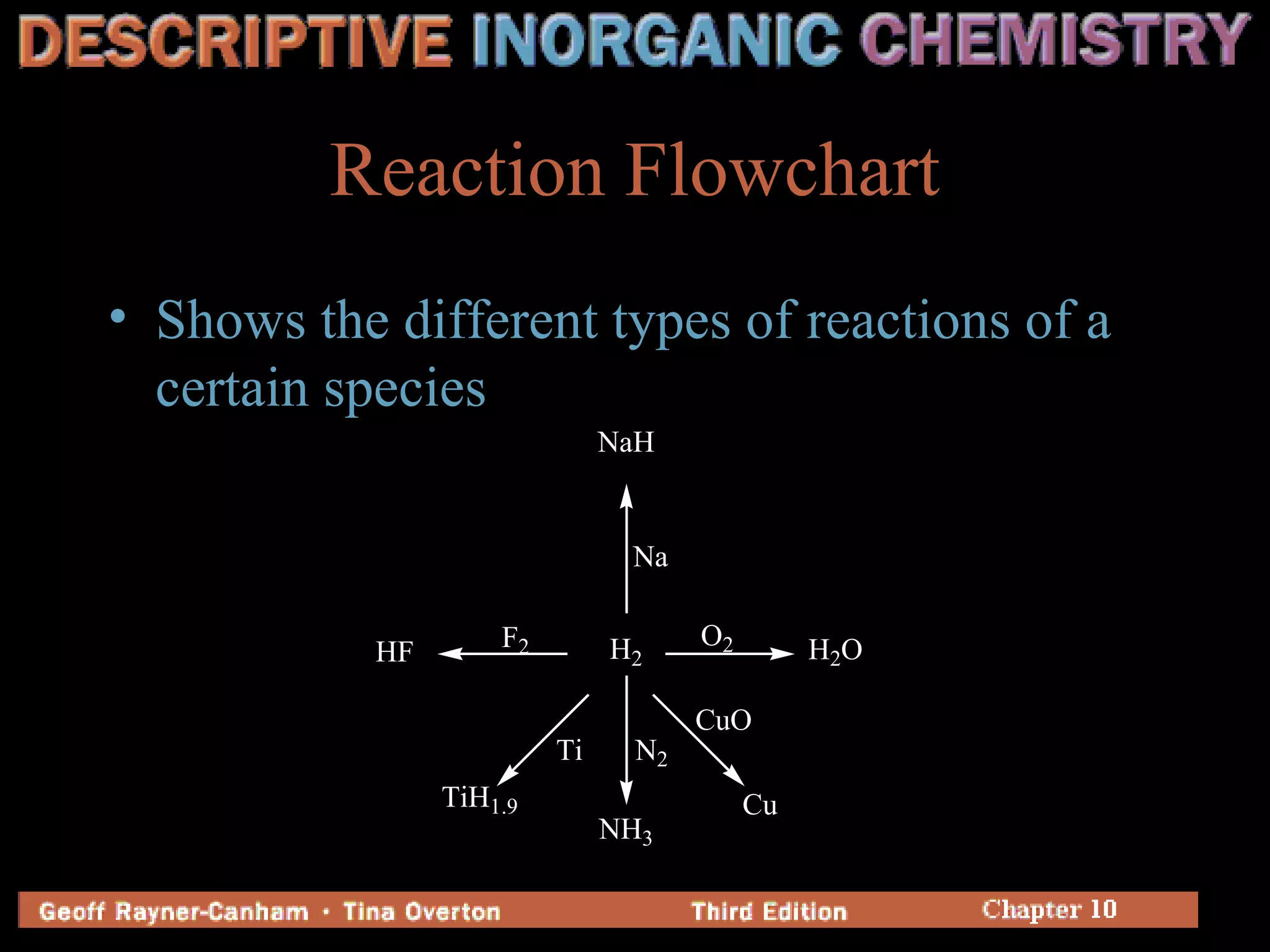
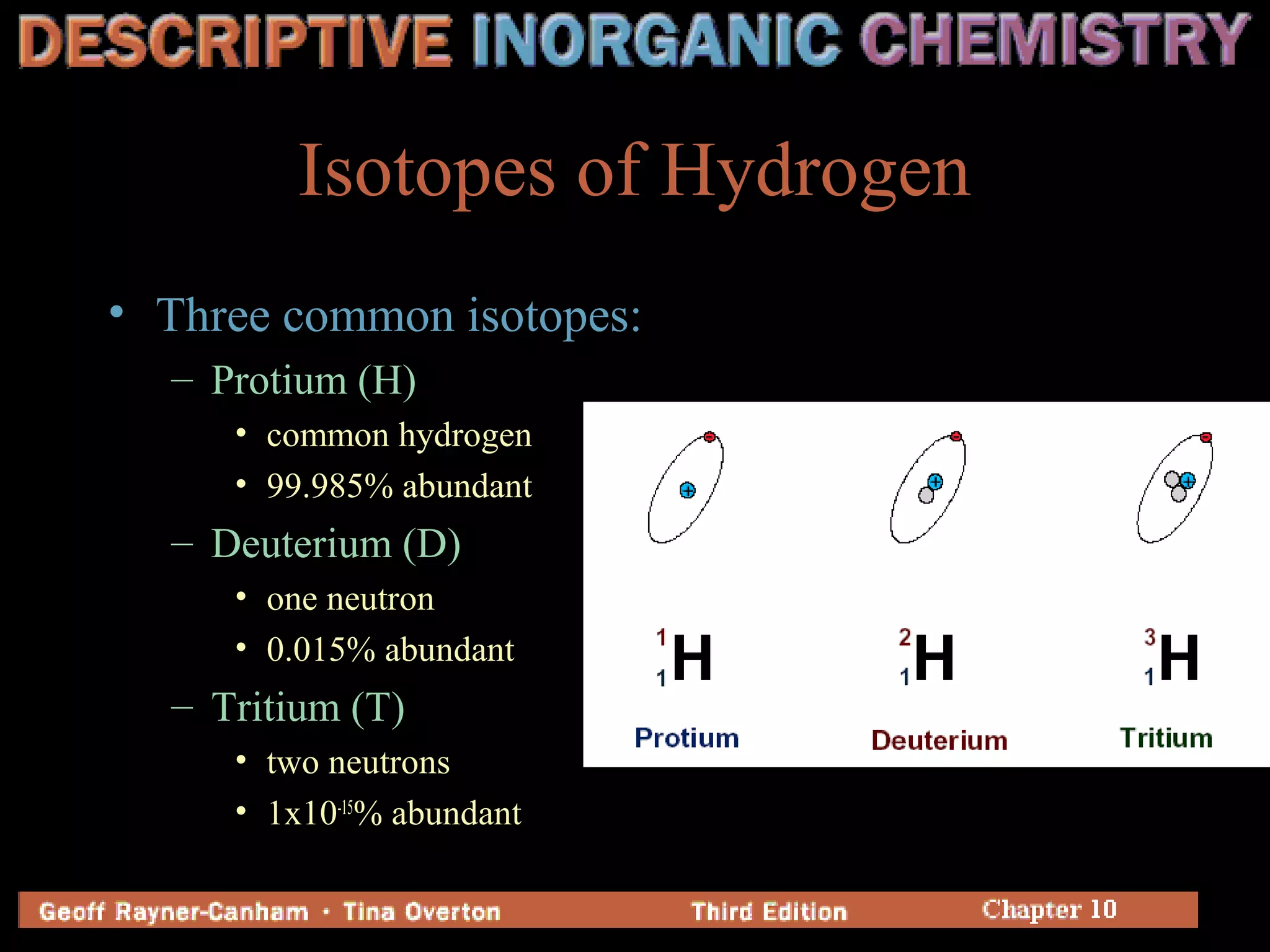
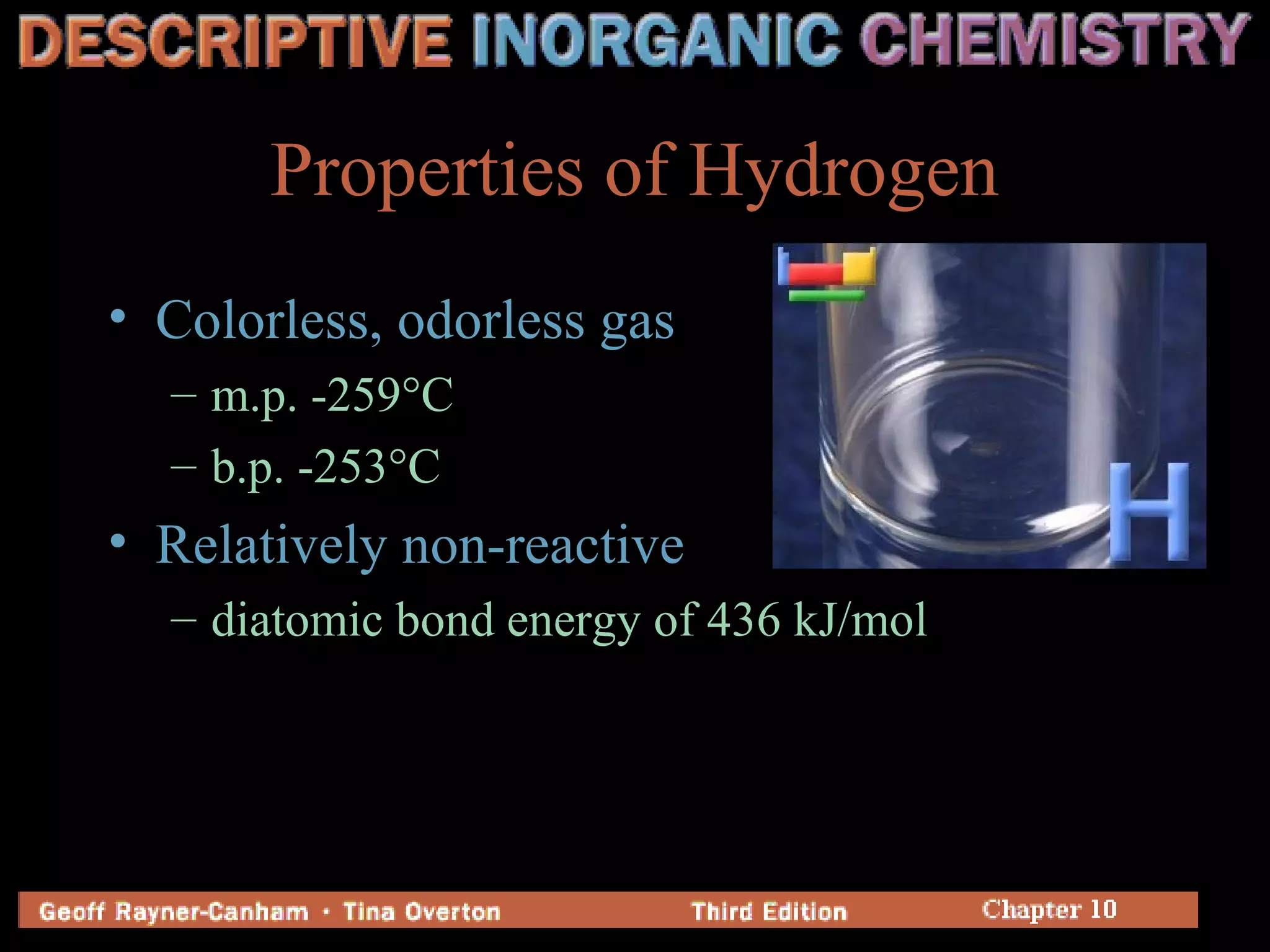
Hydrogen has three common isotopes - protium, deuterium, and tritium. It was discovered over 200 years ago and can exist as a colorless, odorless gas or in compounds such as ionic hydrides, covalent hydrides, and metallic hydrides. Hydrogen bonding plays a key role in water's unusual properties and is important for biological processes like DNA structure.











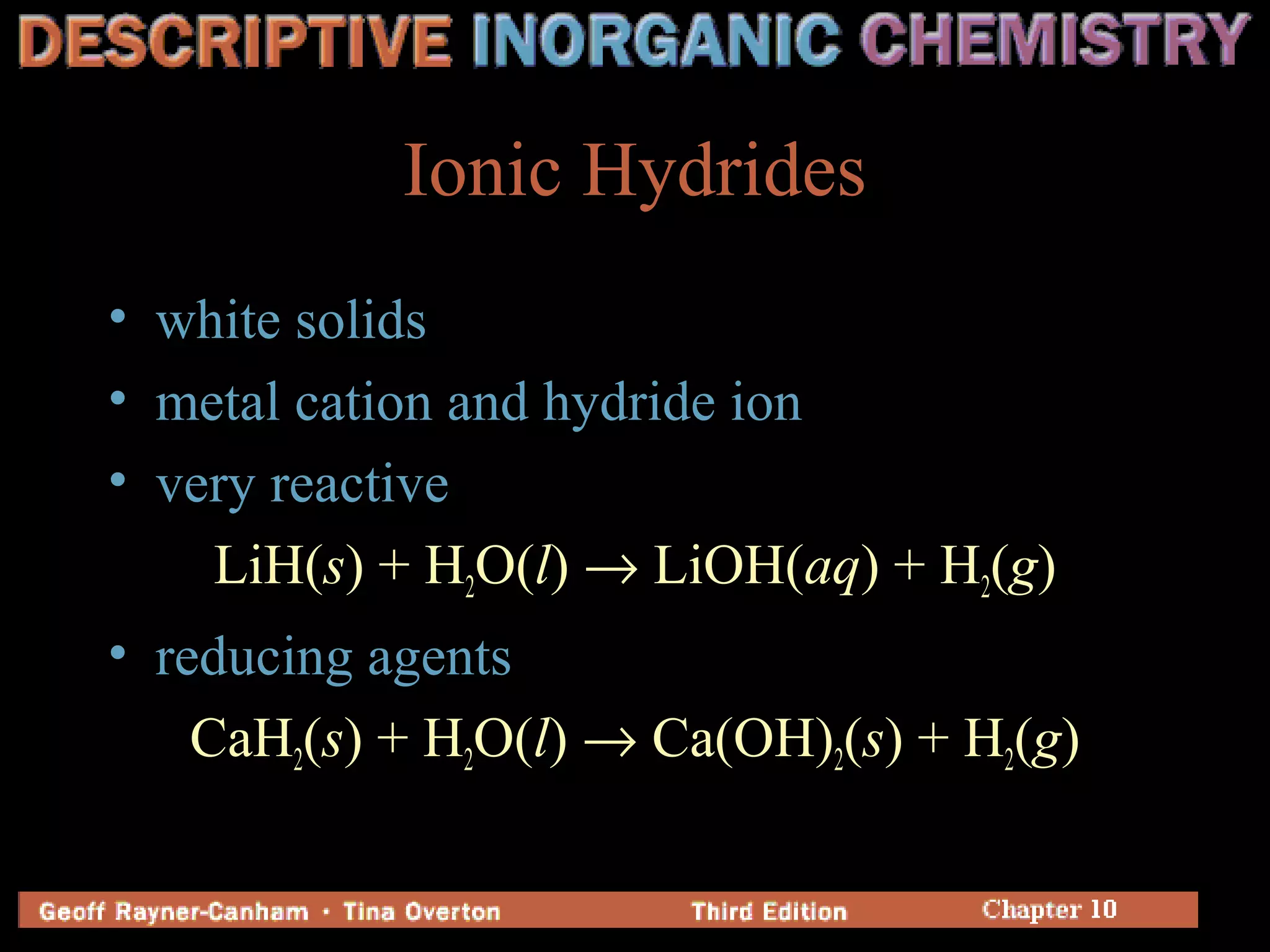








 + H2O(l) + e-
→ [Ni-alloy]H(s) + OH-
(aq)](https://image.slidesharecdn.com/hydrogenppt-171216143241/75/Hydrogen-ppt-29-2048.jpg)