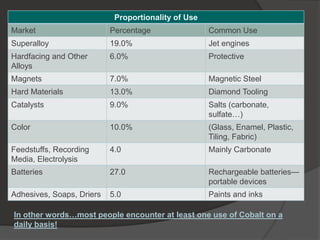
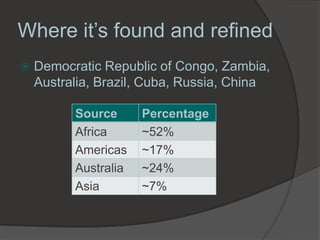
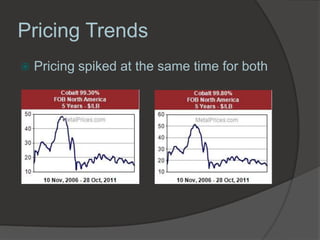
Georg Brandt discovered cobalt in 1739 while attempting to prove that the blue color in glass came from an unknown element, not bismuth as previously thought. Cobalt is a hard, silvery-gray metal that is resistant to corrosion and oxygen. It has many uses including in superalloys for jet engines, magnets, batteries, colorants like blue glass, and medical radiation therapy using radioactive cobalt-60. The Democratic Republic of Congo is the leading producer of cobalt today.