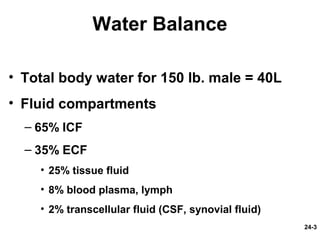
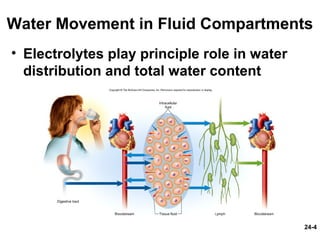
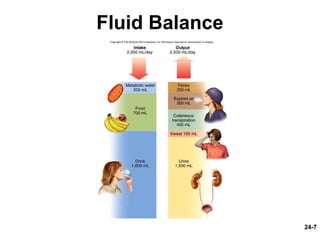
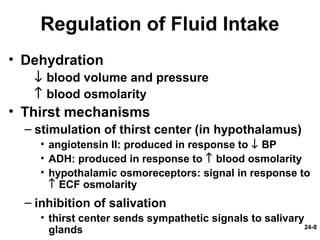
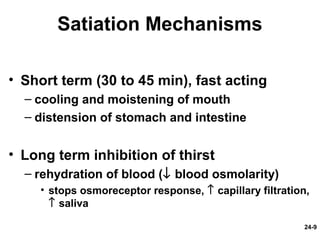
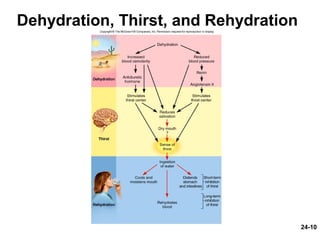
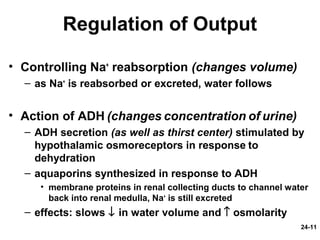
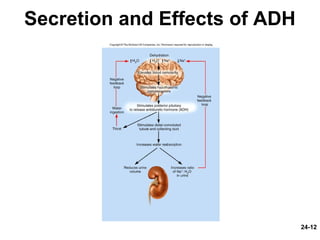
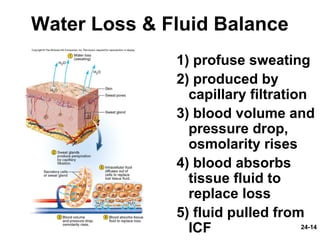
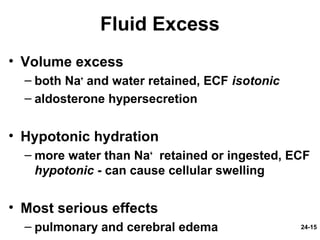
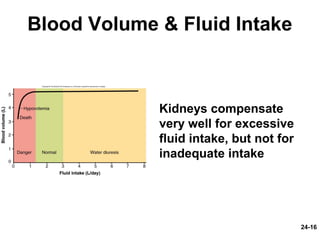
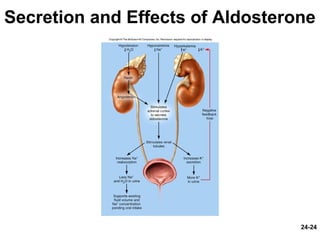
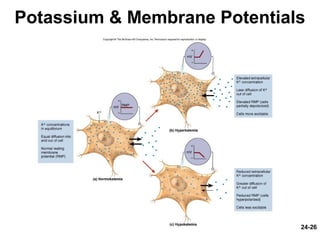
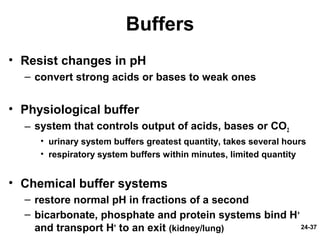
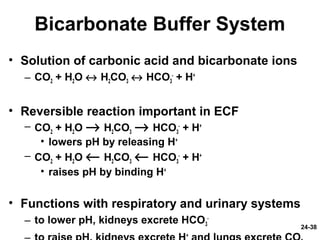
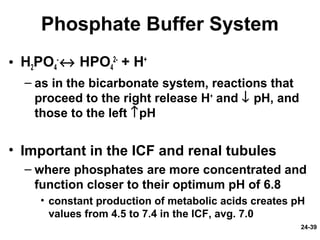
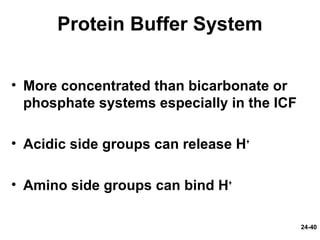
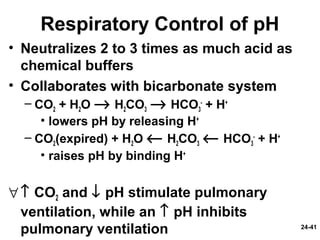
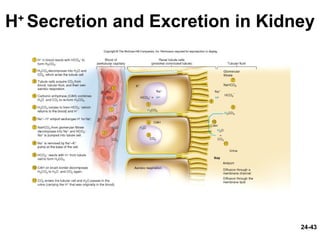
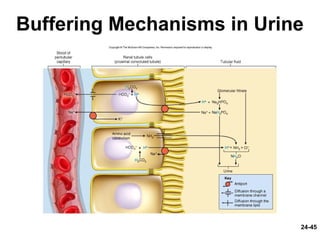
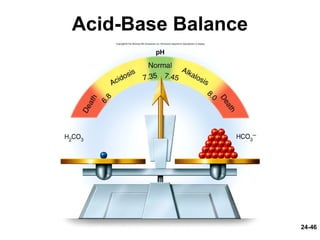
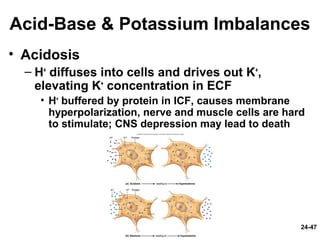
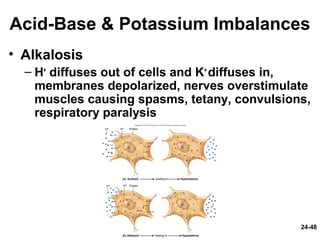
This document provides information on water, electrolyte, and acid-base balance in the human body. It discusses water balance and movement between fluid compartments. It covers mechanisms for regulating fluid intake and output, including the roles of thirst, ADH, and aldosterone. Disorders of water balance like dehydration and fluid excess are described. The functions and homeostasis of major electrolytes sodium, potassium, chloride, and calcium are outlined. Buffering systems including bicarbonate, phosphate and proteins that help regulate acid-base balance are also summarized.