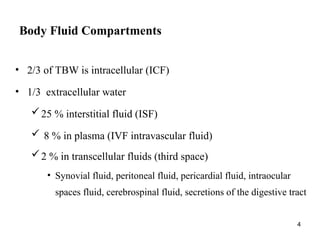
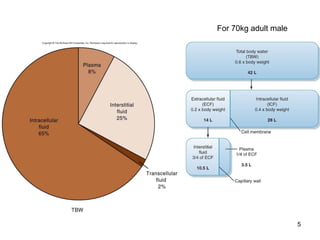
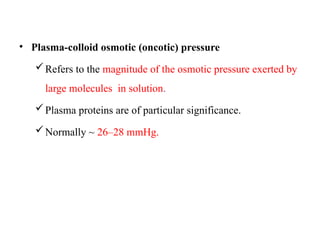
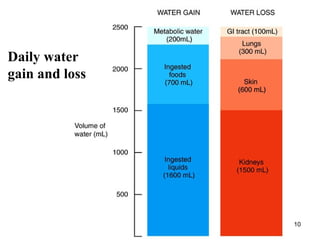
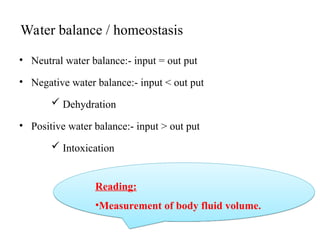
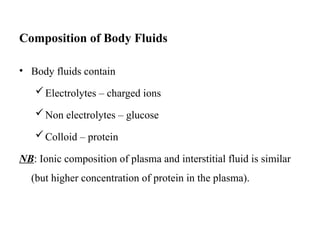
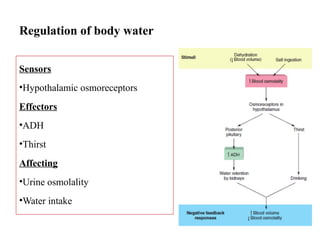
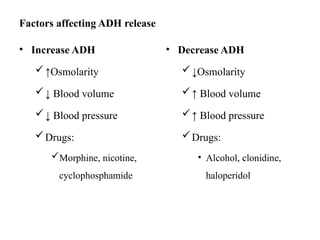
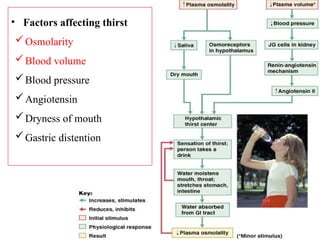
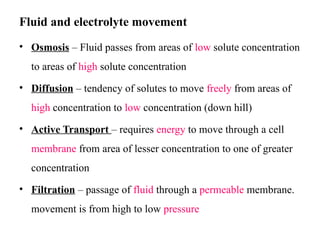
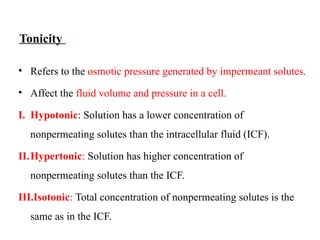
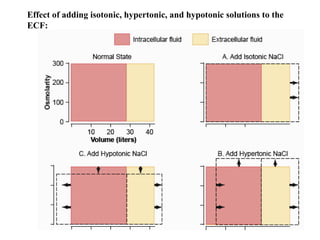
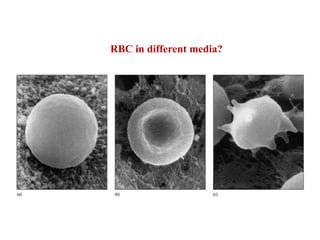
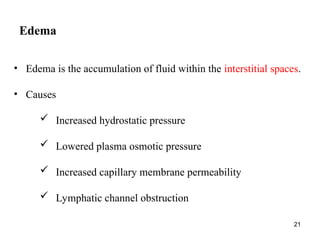
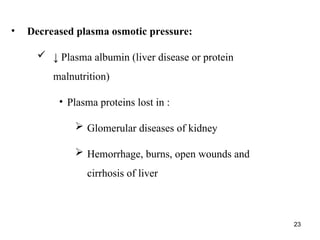
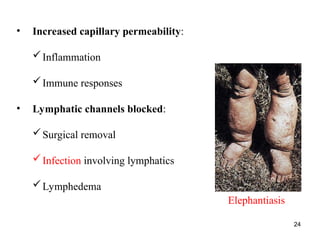
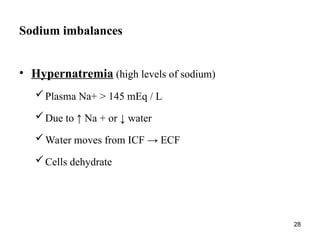
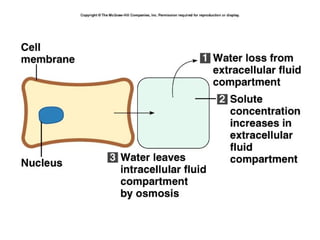
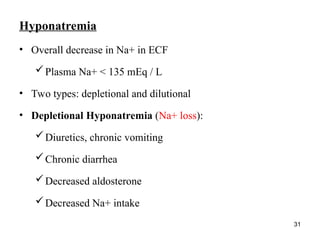
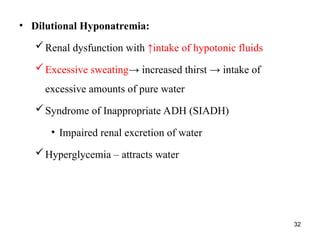
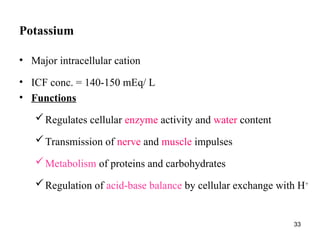
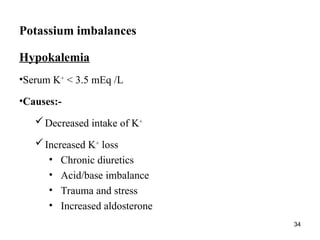
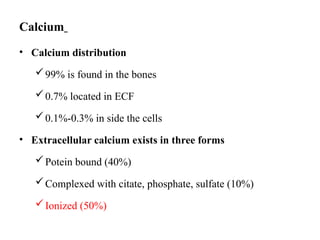
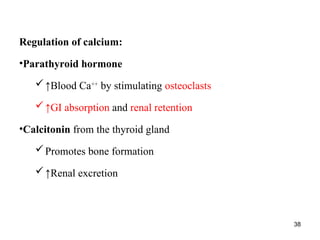
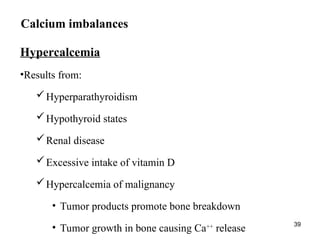
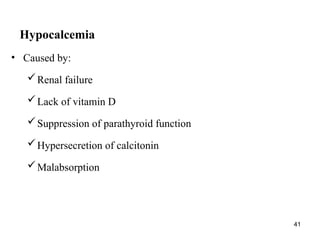
Water serves vital functions in the body, including transporting nutrients and waste, regulating temperature, and facilitating metabolism. Body composition varies by factors such as fat, age, and sex, with water content being approximately 60% in adults. Electrolyte balance is crucial, with sodium and potassium being key players; imbalances can lead to severe health issues.