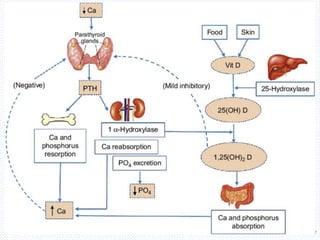
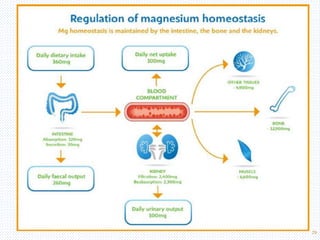
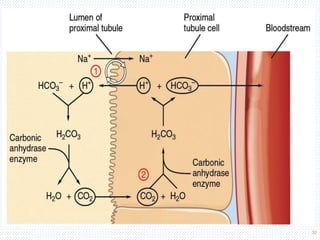
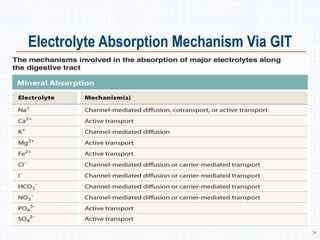
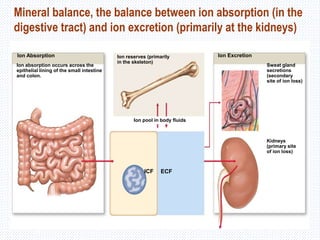
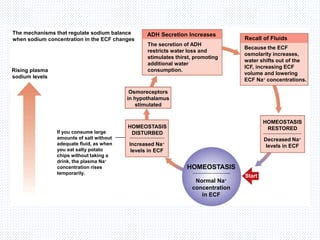
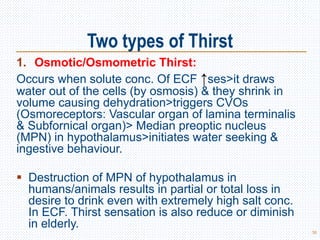
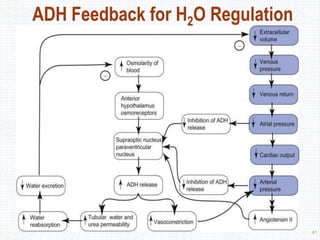
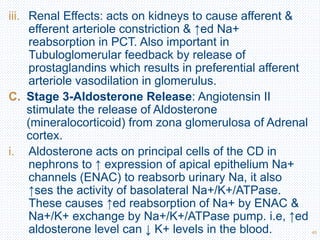
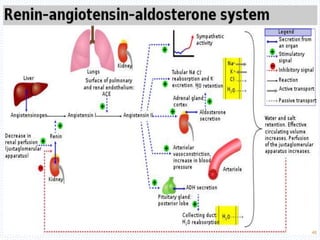
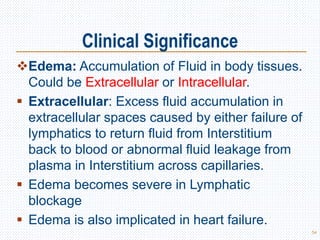
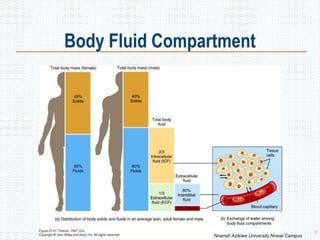
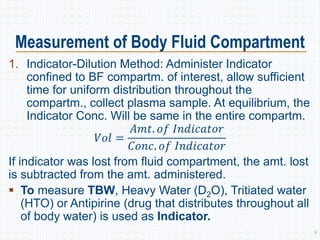
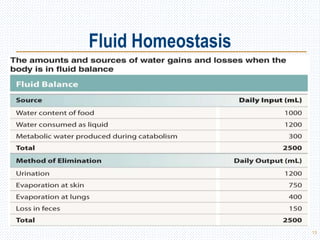
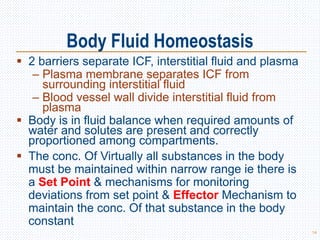
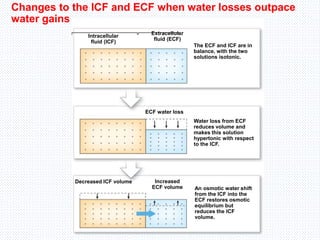
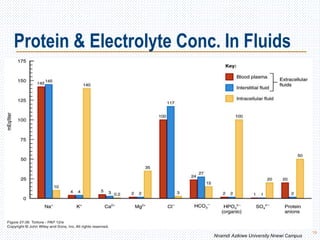
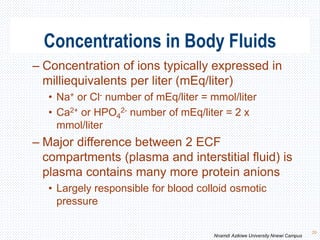
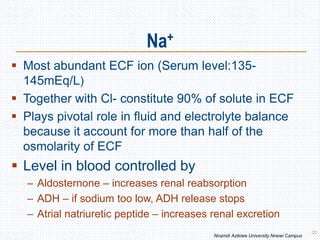
This document discusses body fluids and electrolyte balance. It begins by outlining the major body fluid compartments - intracellular fluid, interstitial fluid, and plasma. The functions of body fluids are then described. Key points include body fluids acting as a medium for metabolic reactions and transporting nutrients and waste. The document outlines various indicators used to measure body fluid compartments and discusses homeostasis of body fluids and electrolytes. Major electrolytes like sodium, potassium, calcium, and chloride are explained in depth, including their roles, concentrations in different fluid compartments, and regulatory mechanisms.


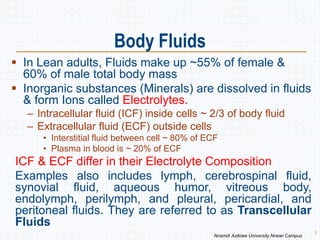












![Factors That Alter K+ Distribution Between Fluid
Compartments
Factors That Shift K+ into
Cells
(Decrease Extracellular [K+])
Factors That Shift K+ Out of Cells
(Increase Extracellular [K+])
Insulin Insulin deficiency (DM)
Aldosterone Aldosterone deficiency
b-adrenergic stimulation b-adrenergic blockade
Alkalosis Acidosis
Cell lysis
↑ed ECF Osmolarity
Strenuous exercise 25](https://image.slidesharecdn.com/davidonbodyfluidsbalance-210430064504/85/Body-Fluids-and-Electrolyte-Homeostasis-25-320.jpg)