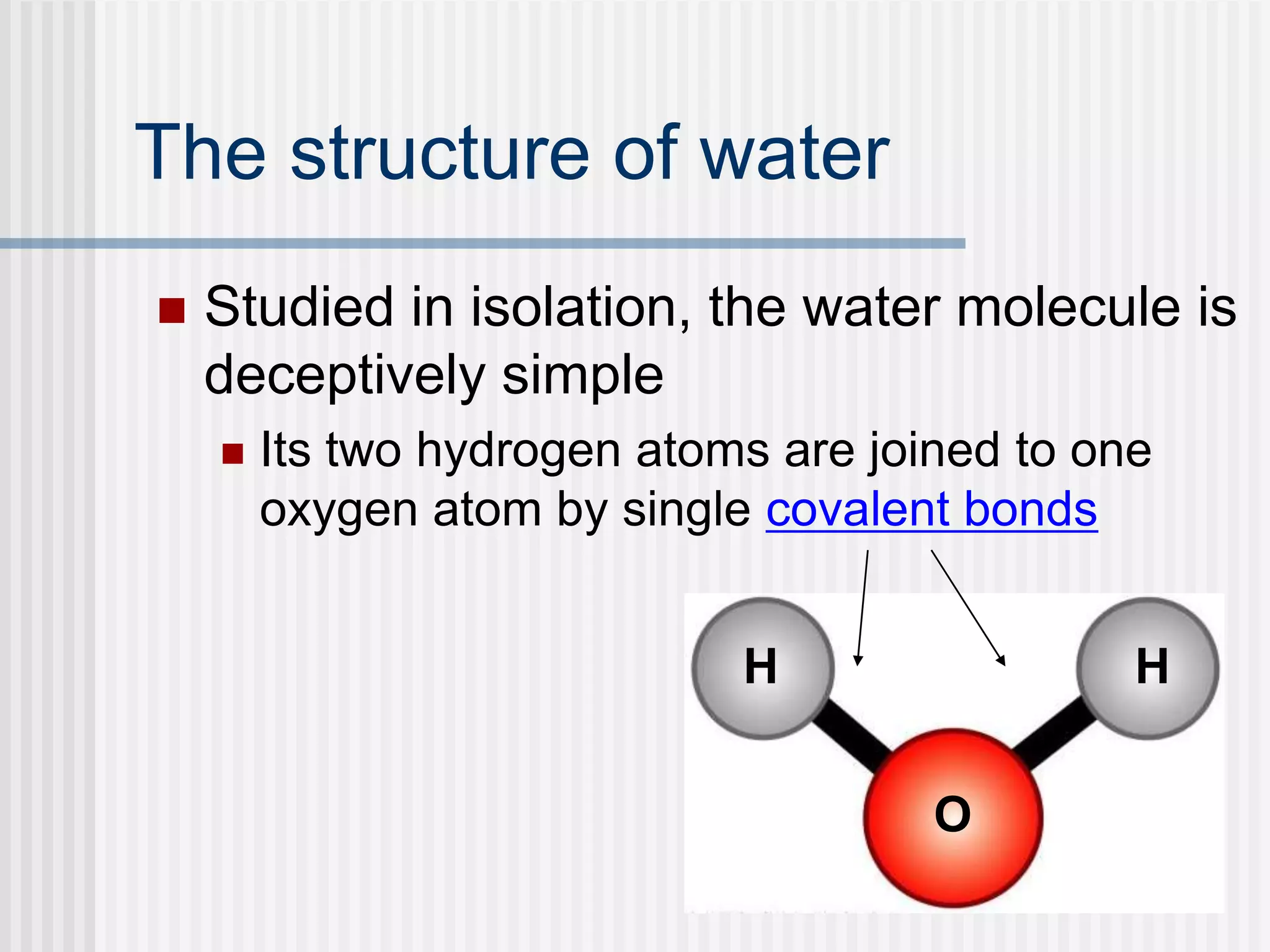
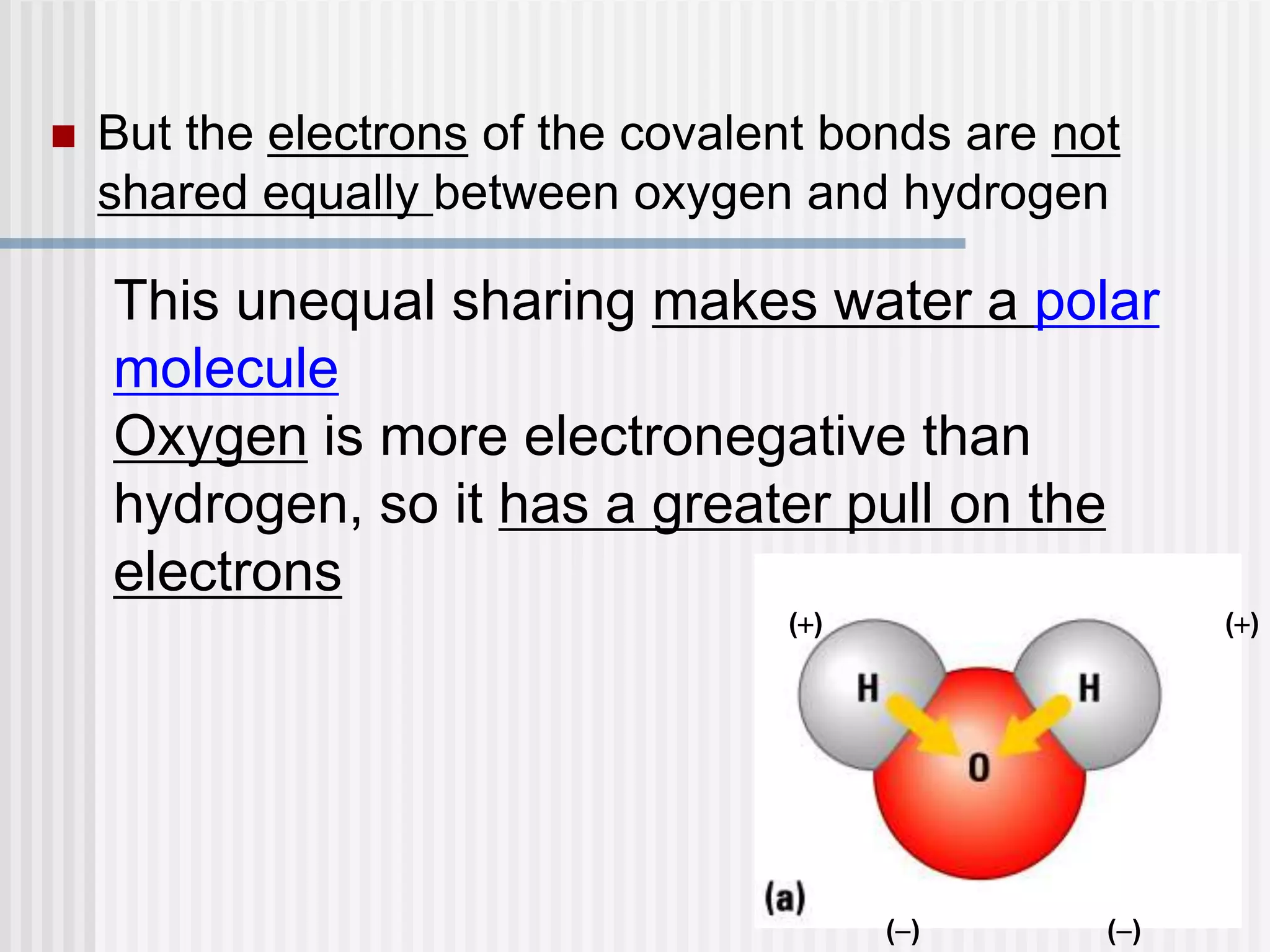
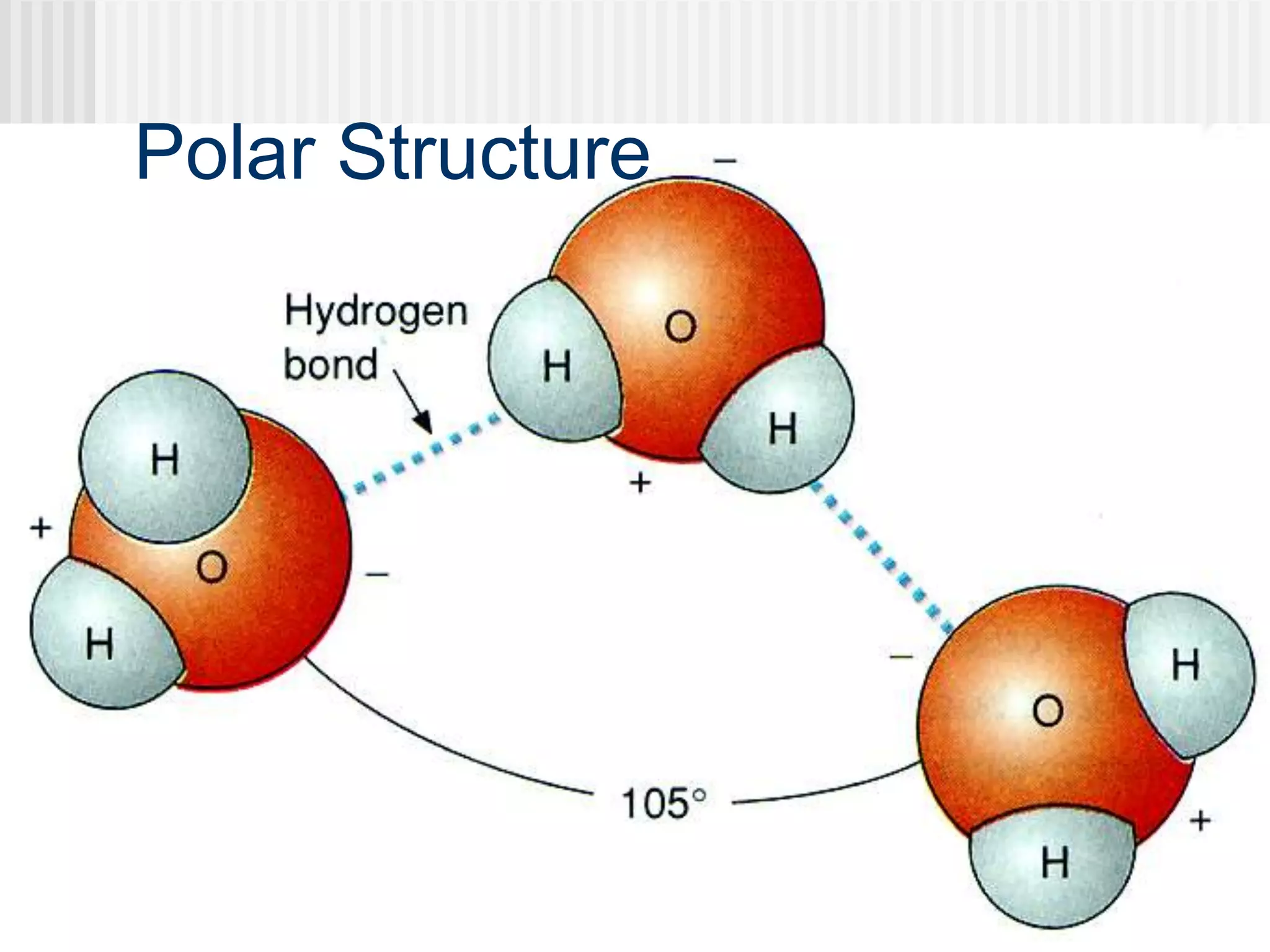
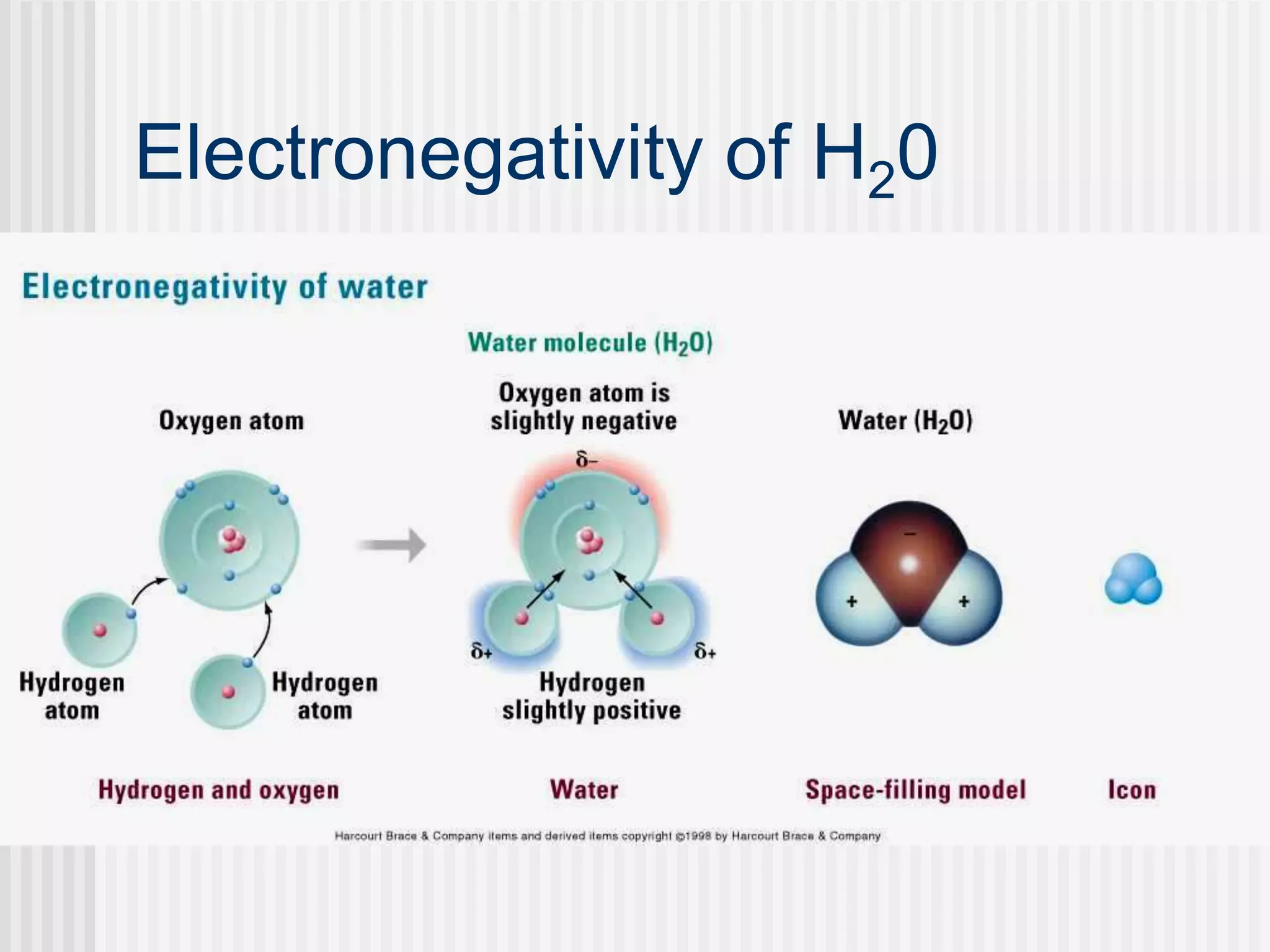
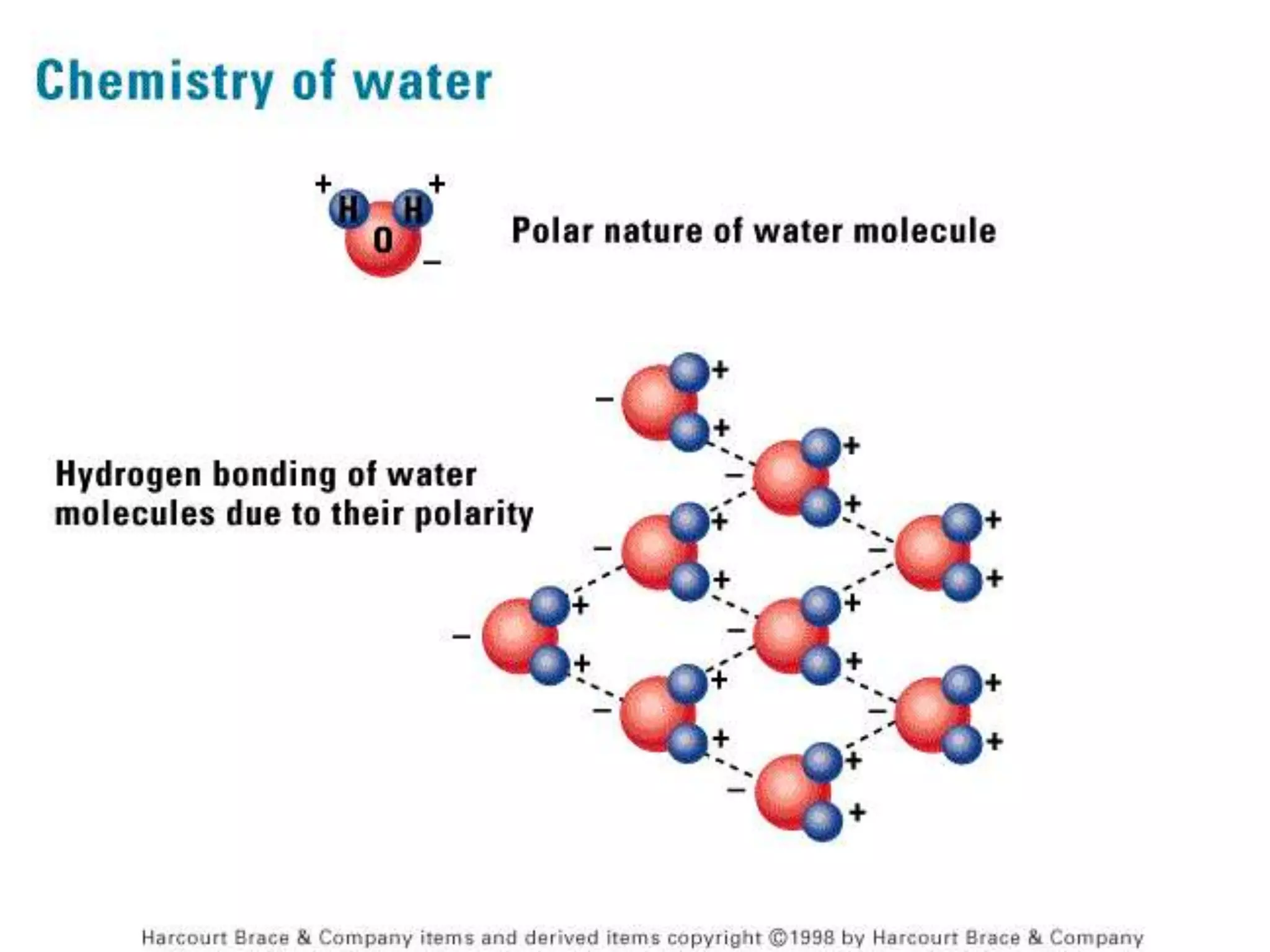
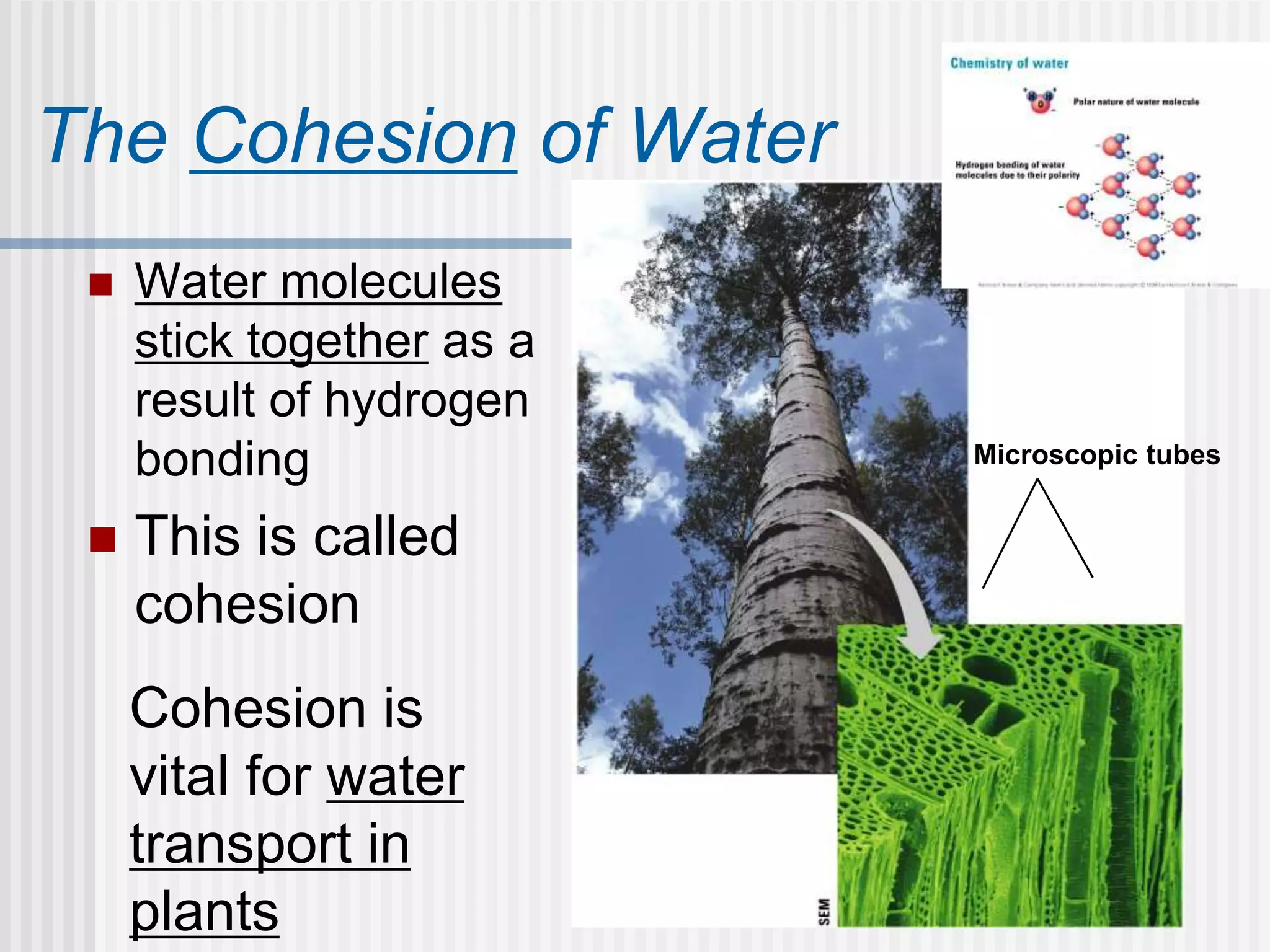
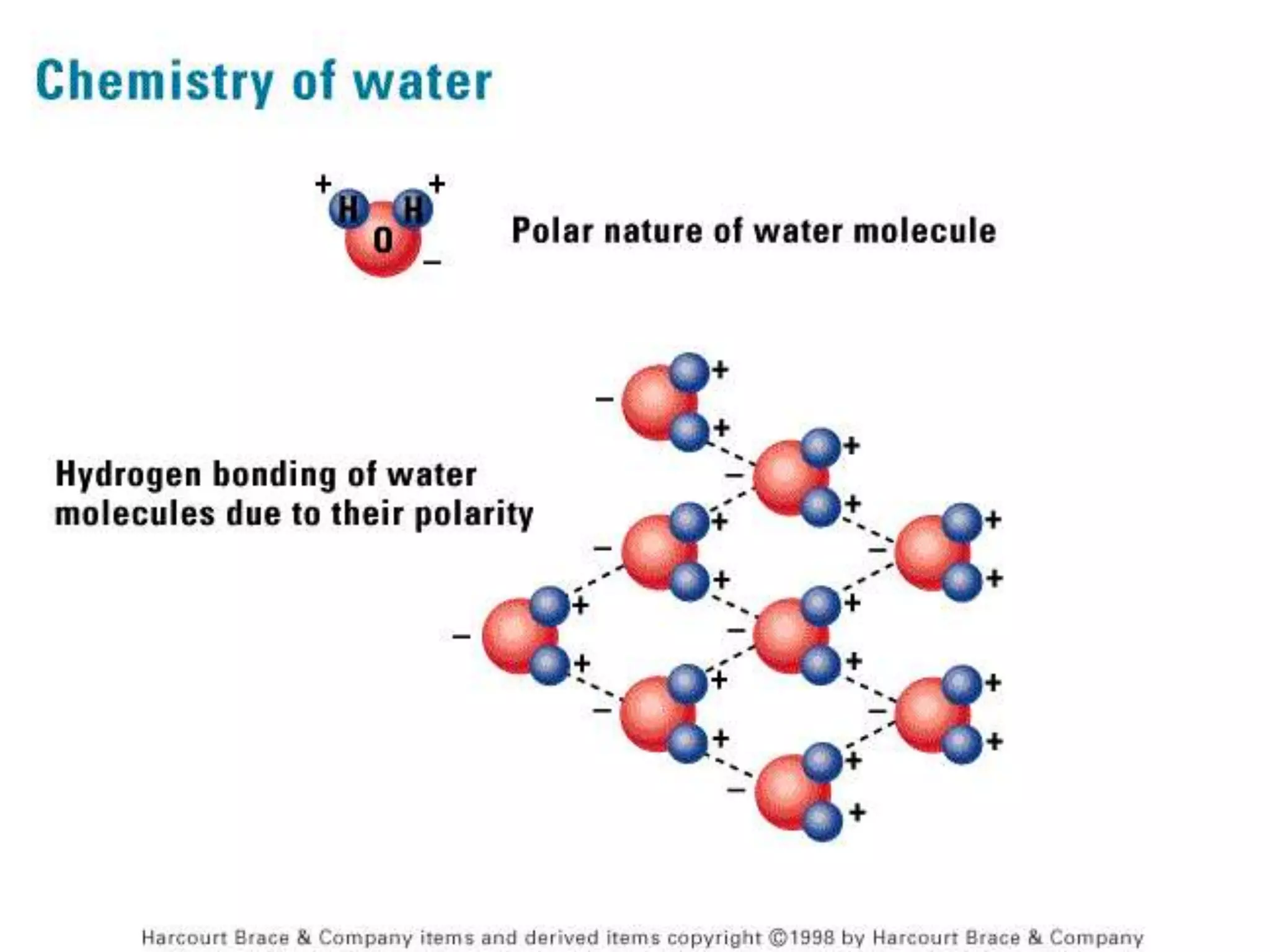
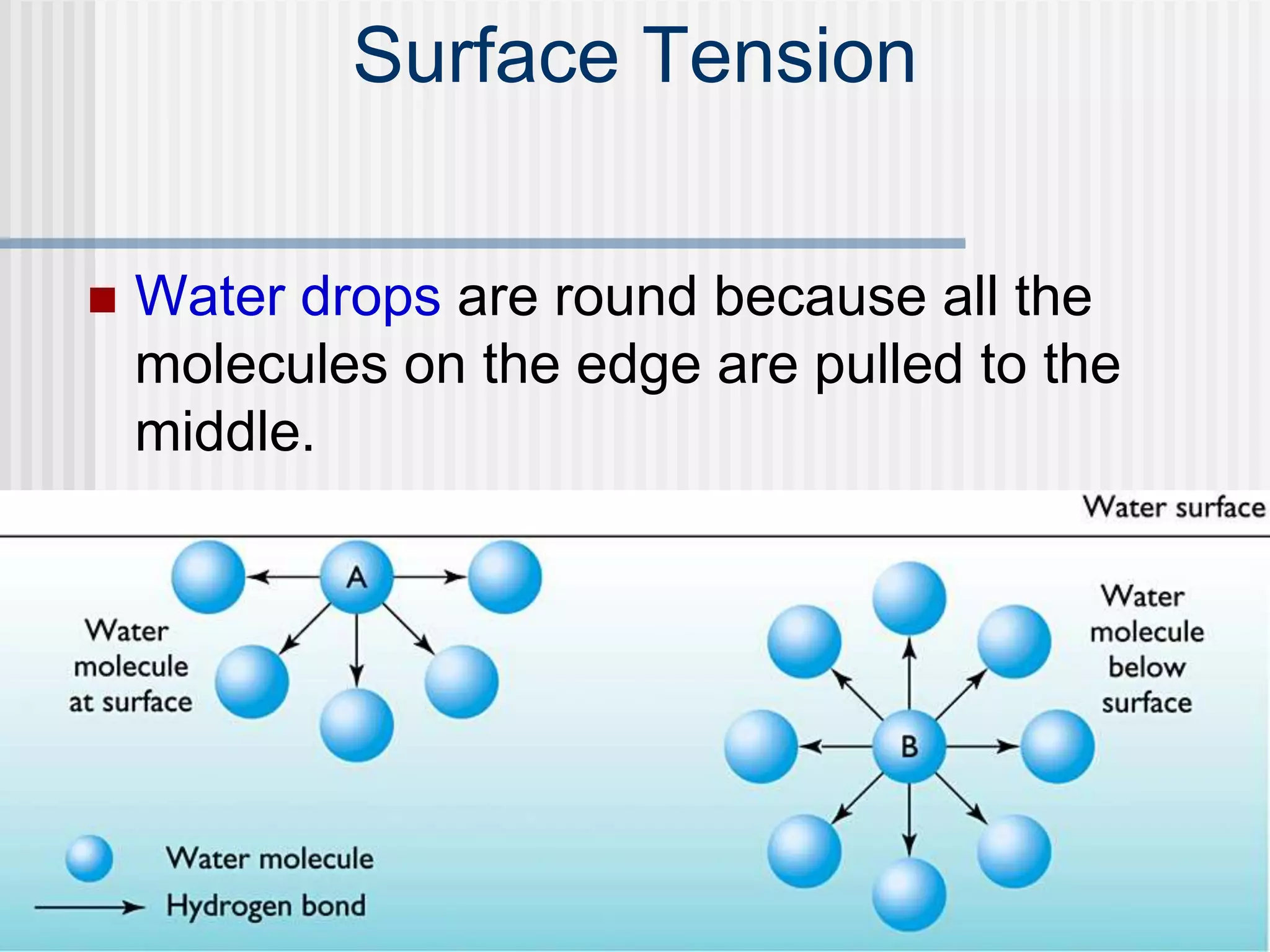
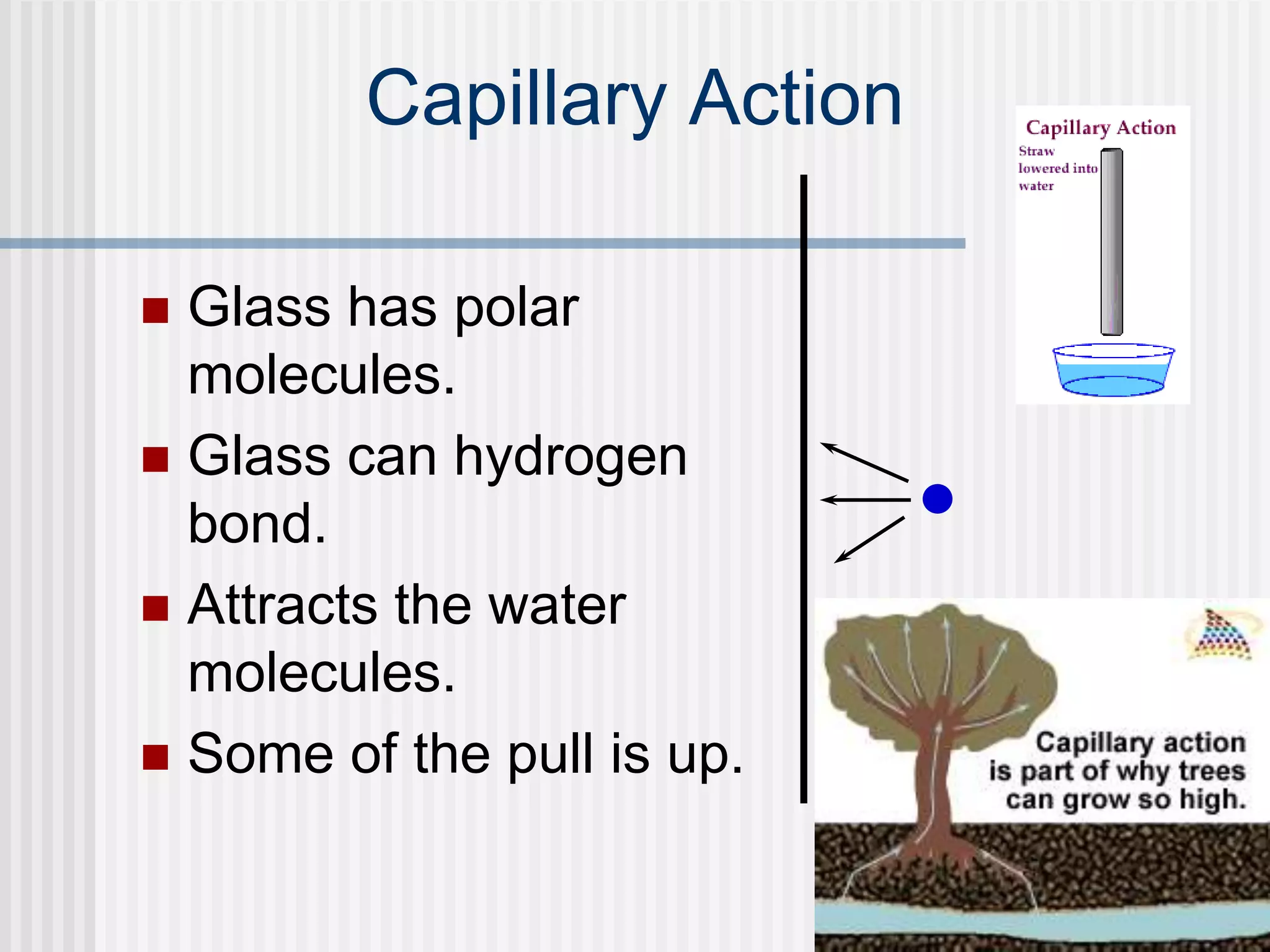
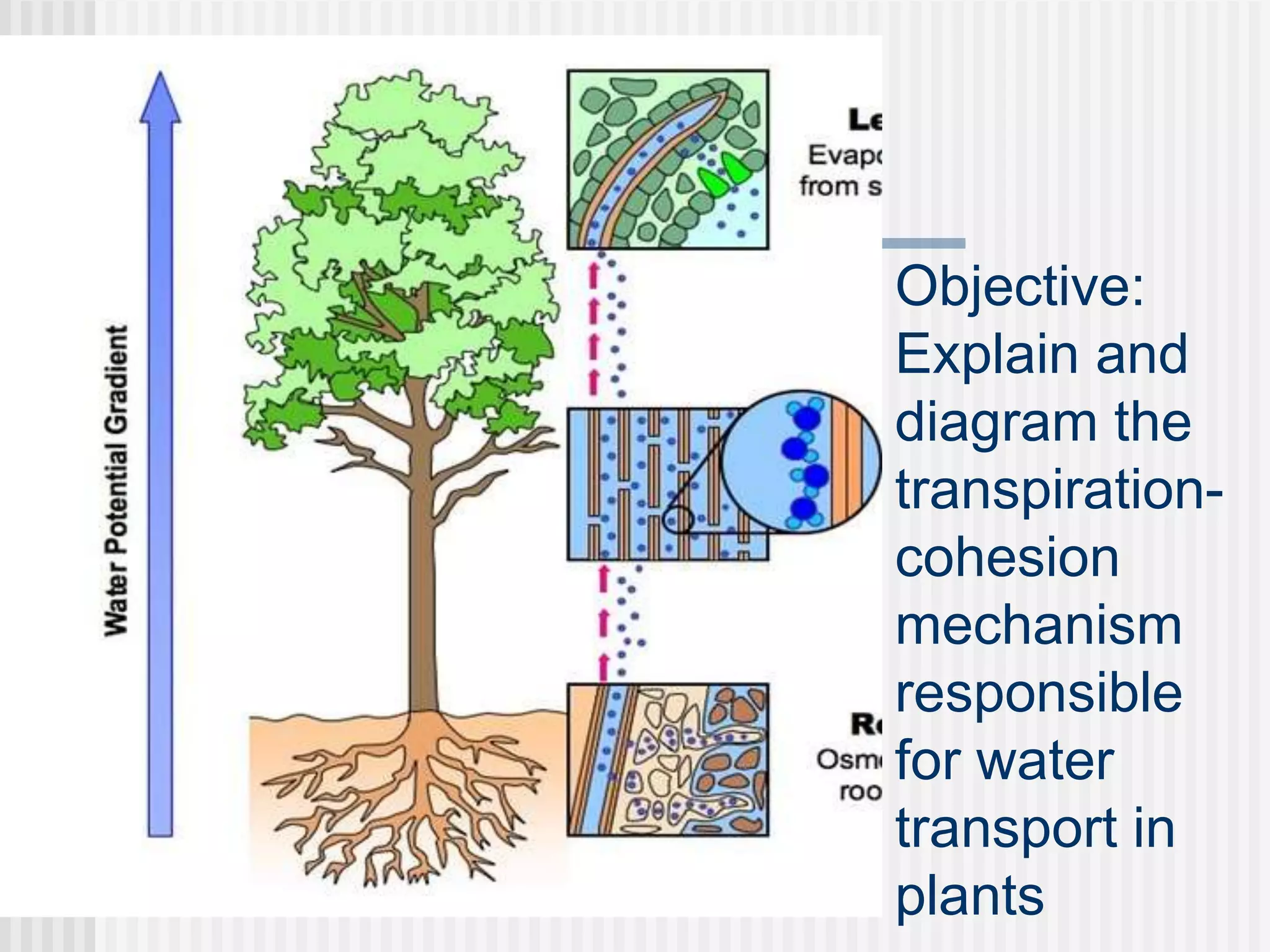
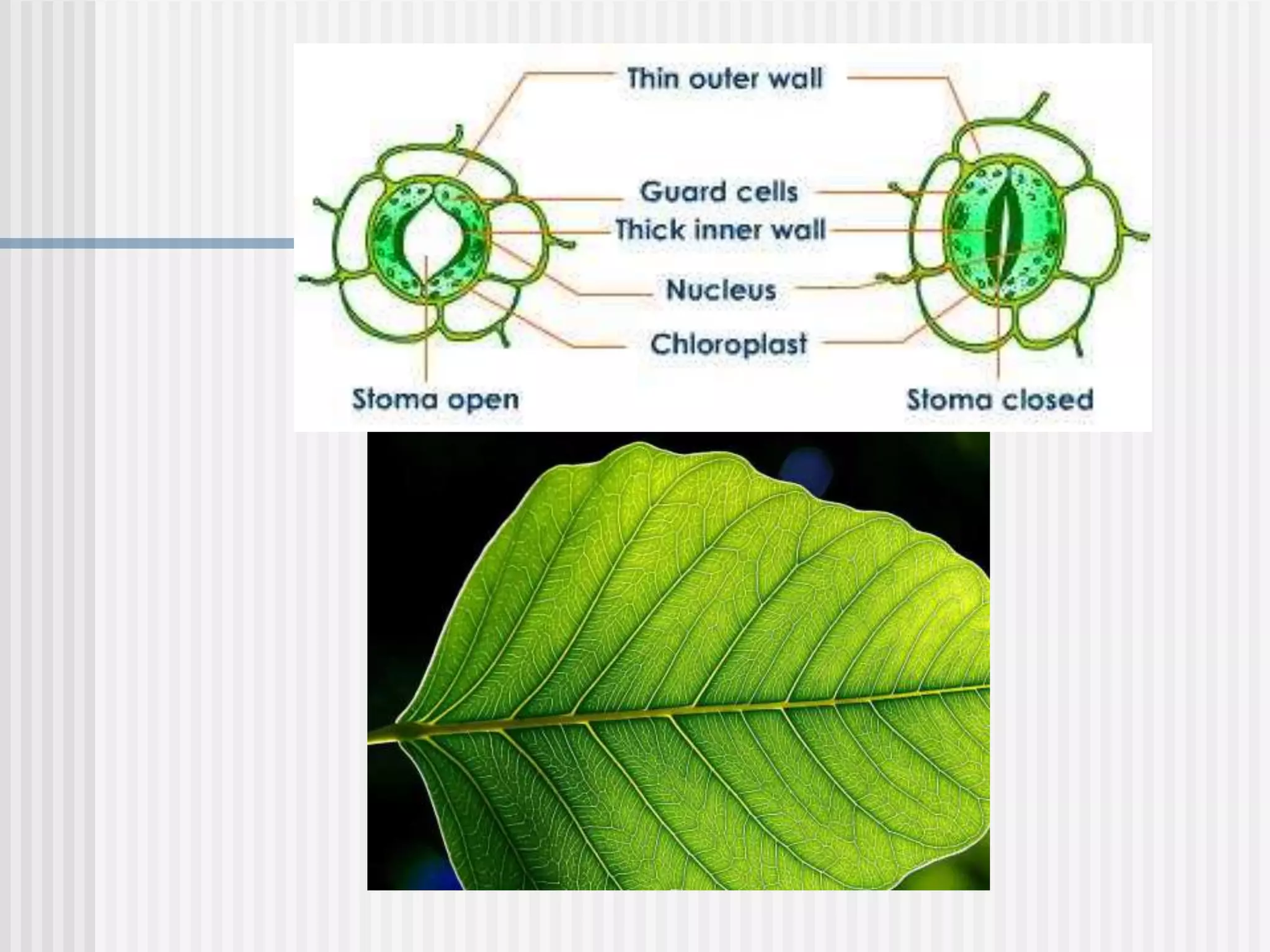
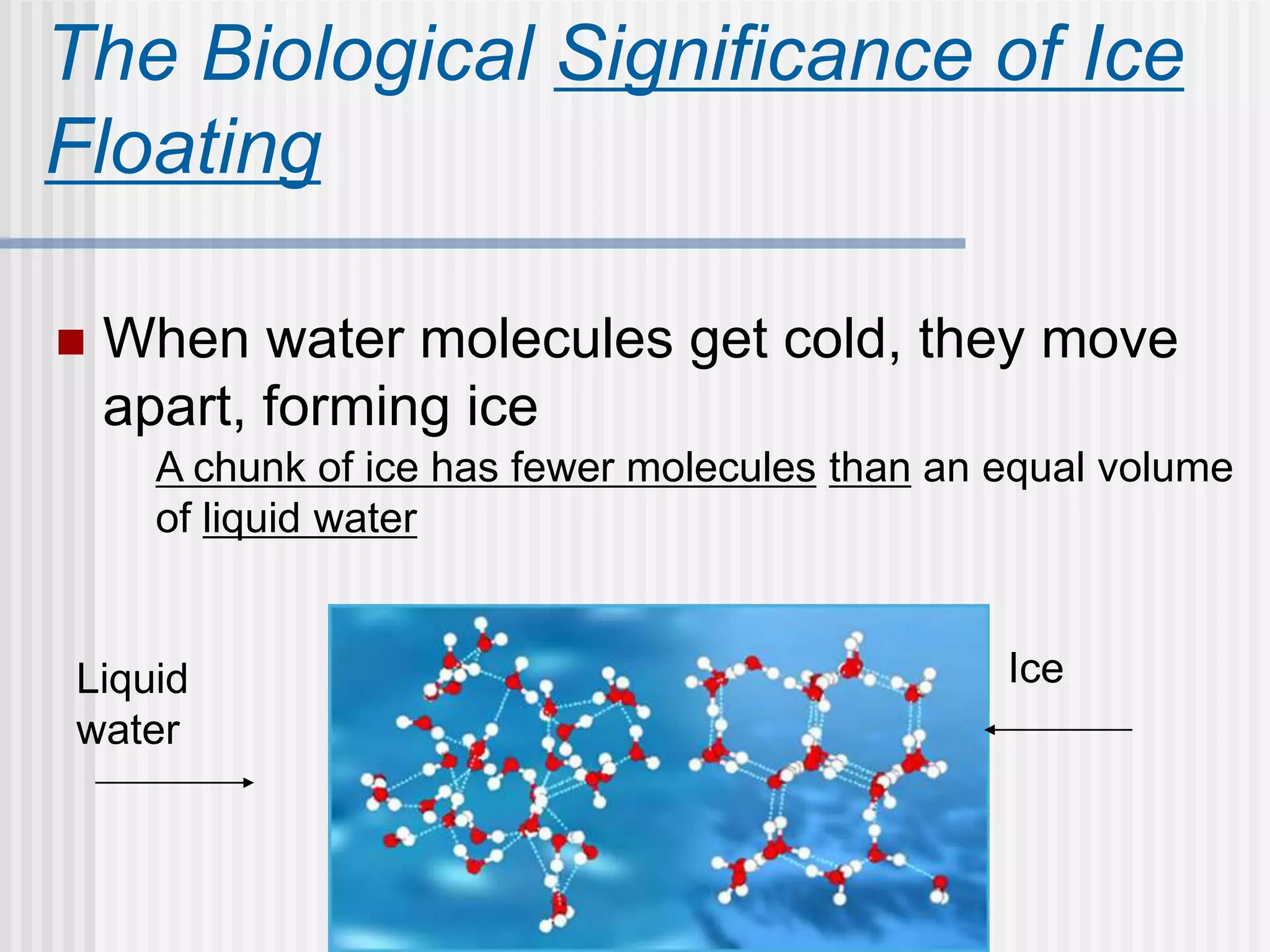
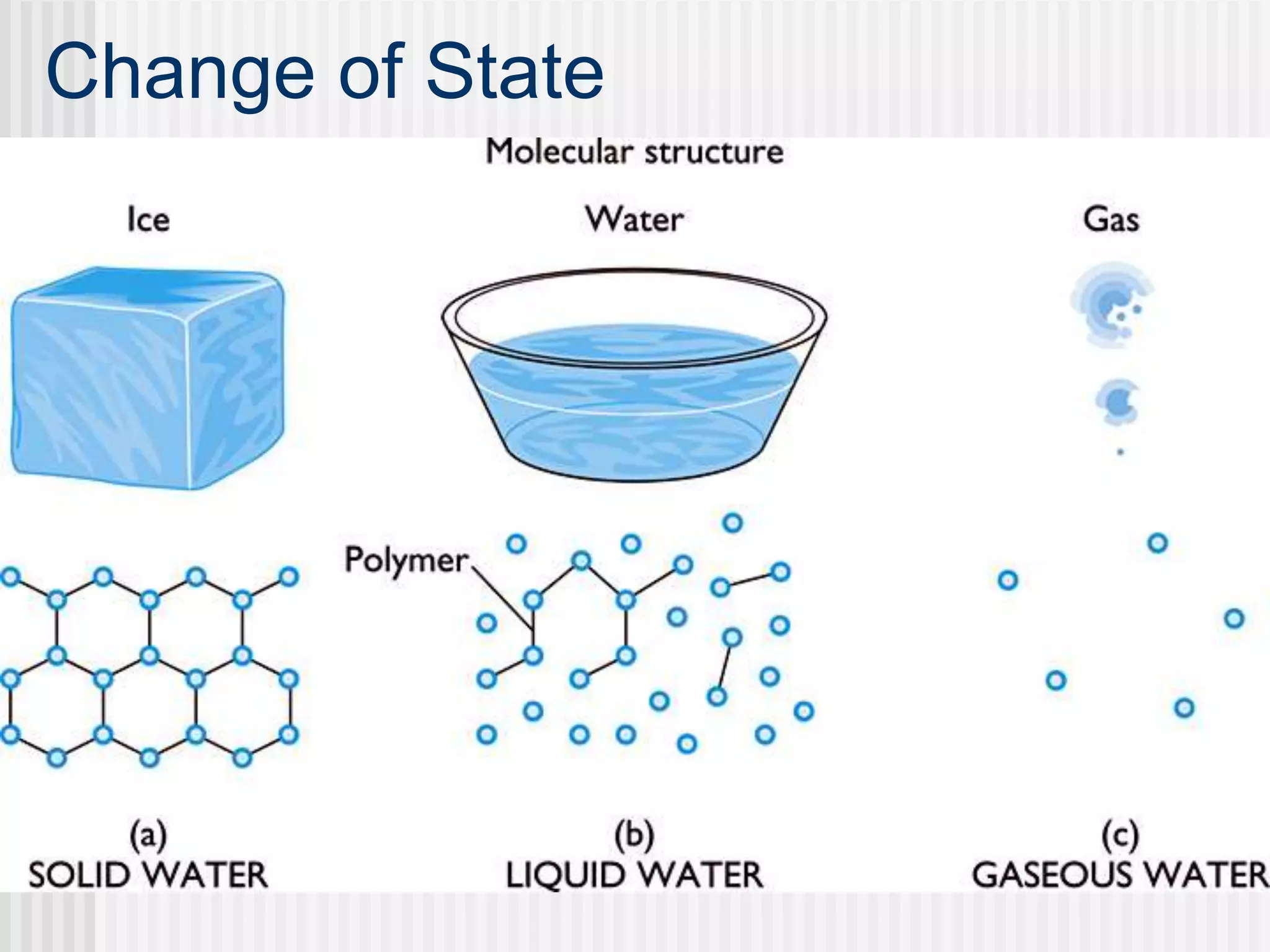
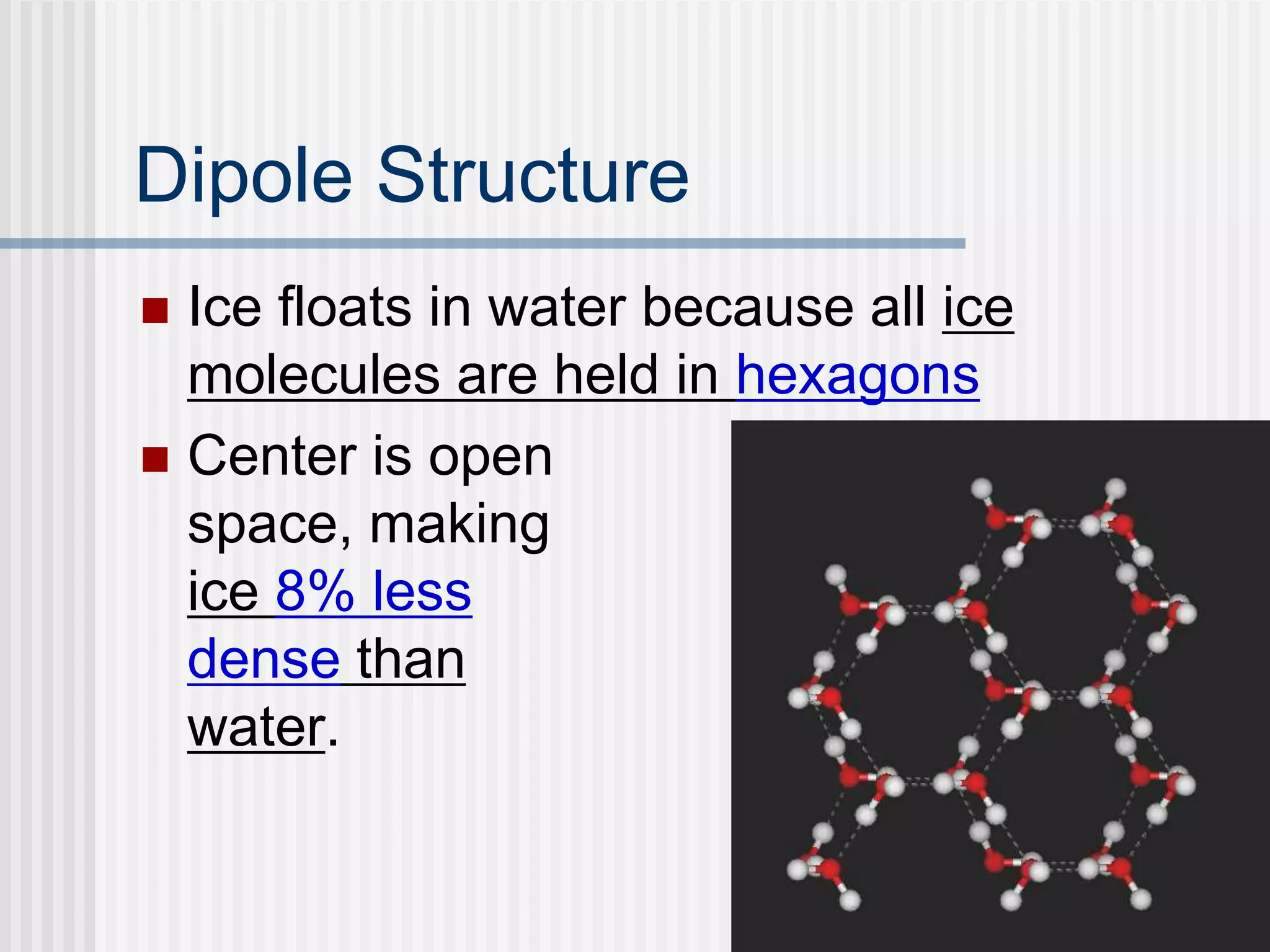
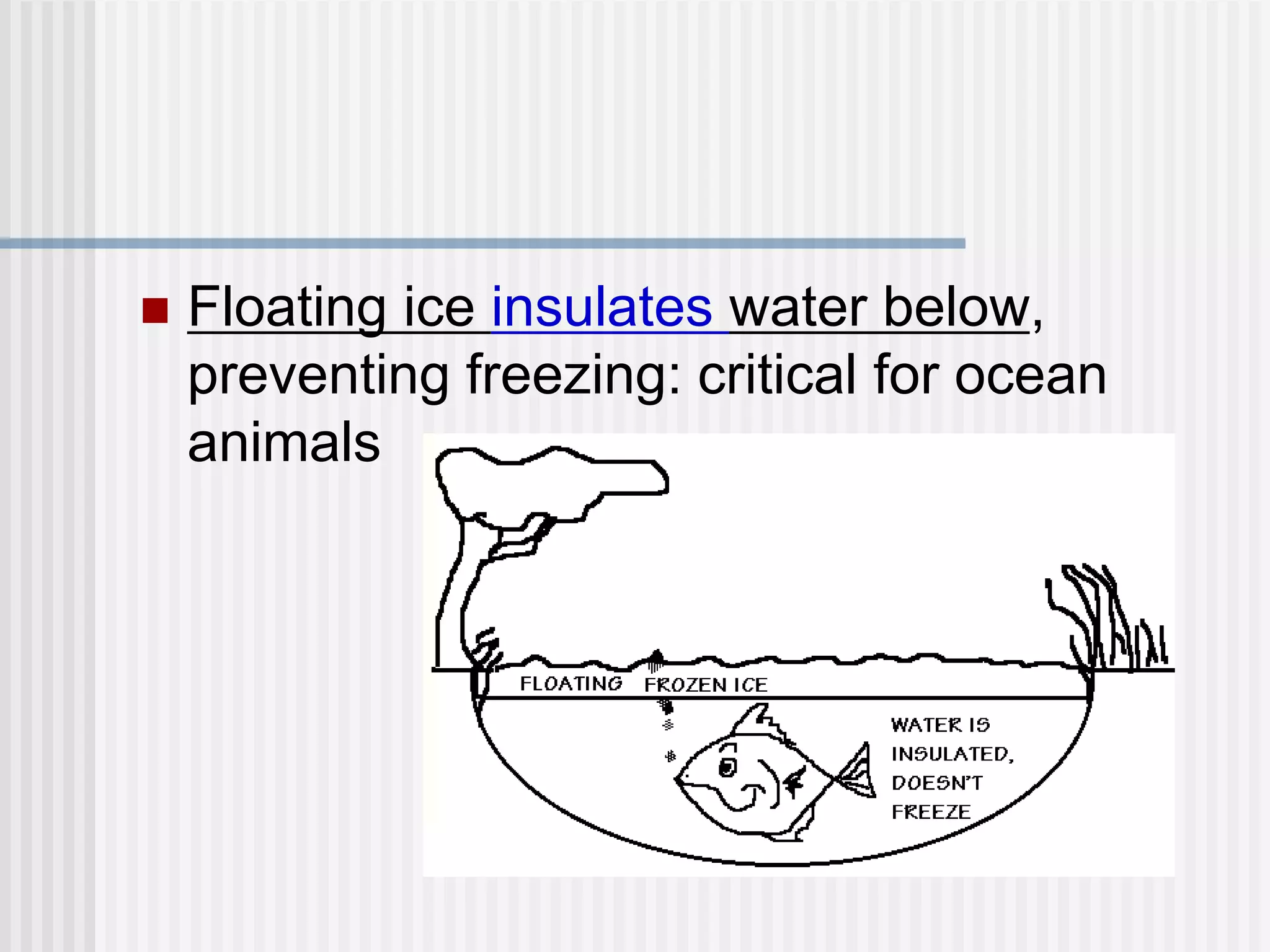
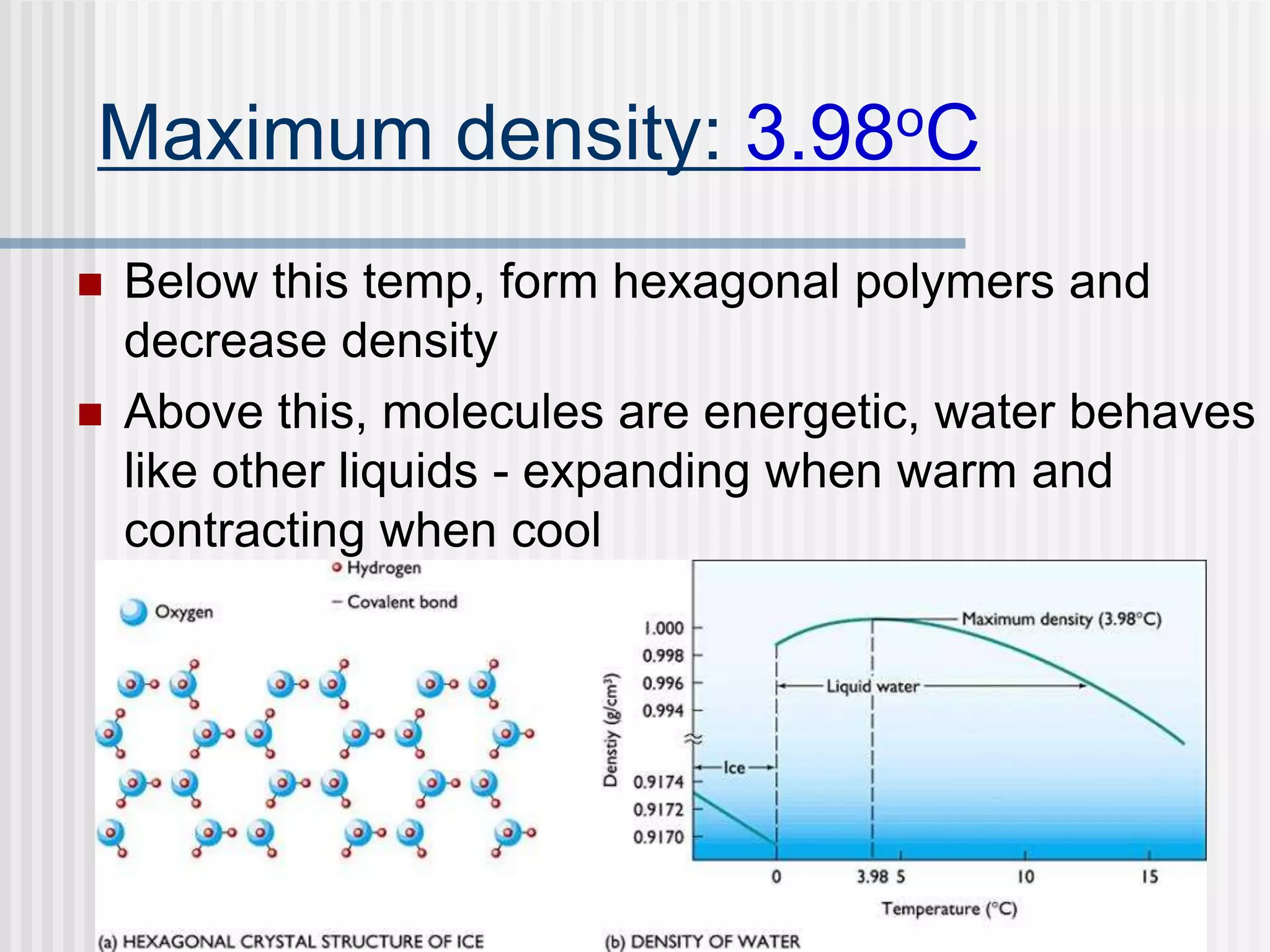
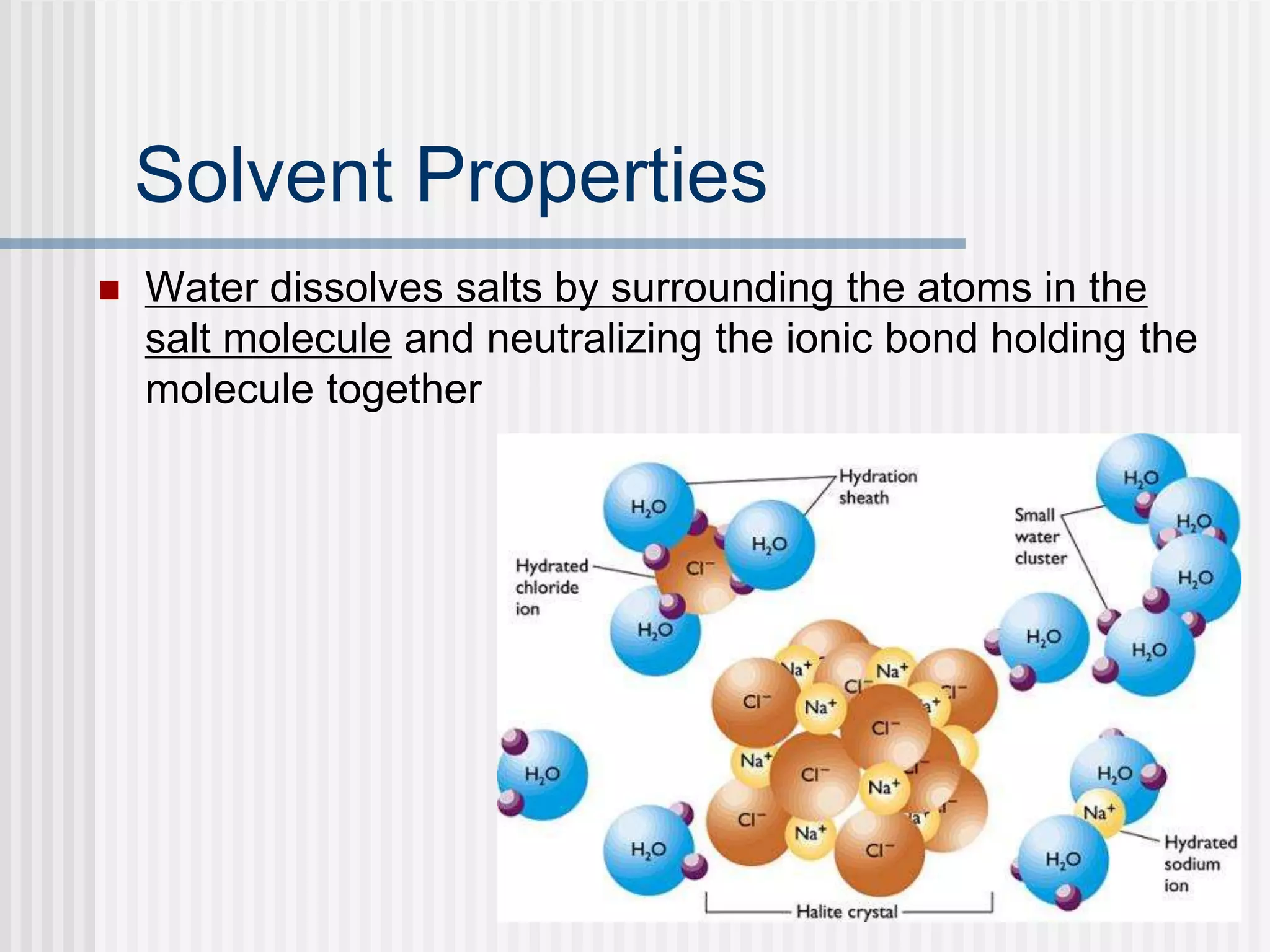
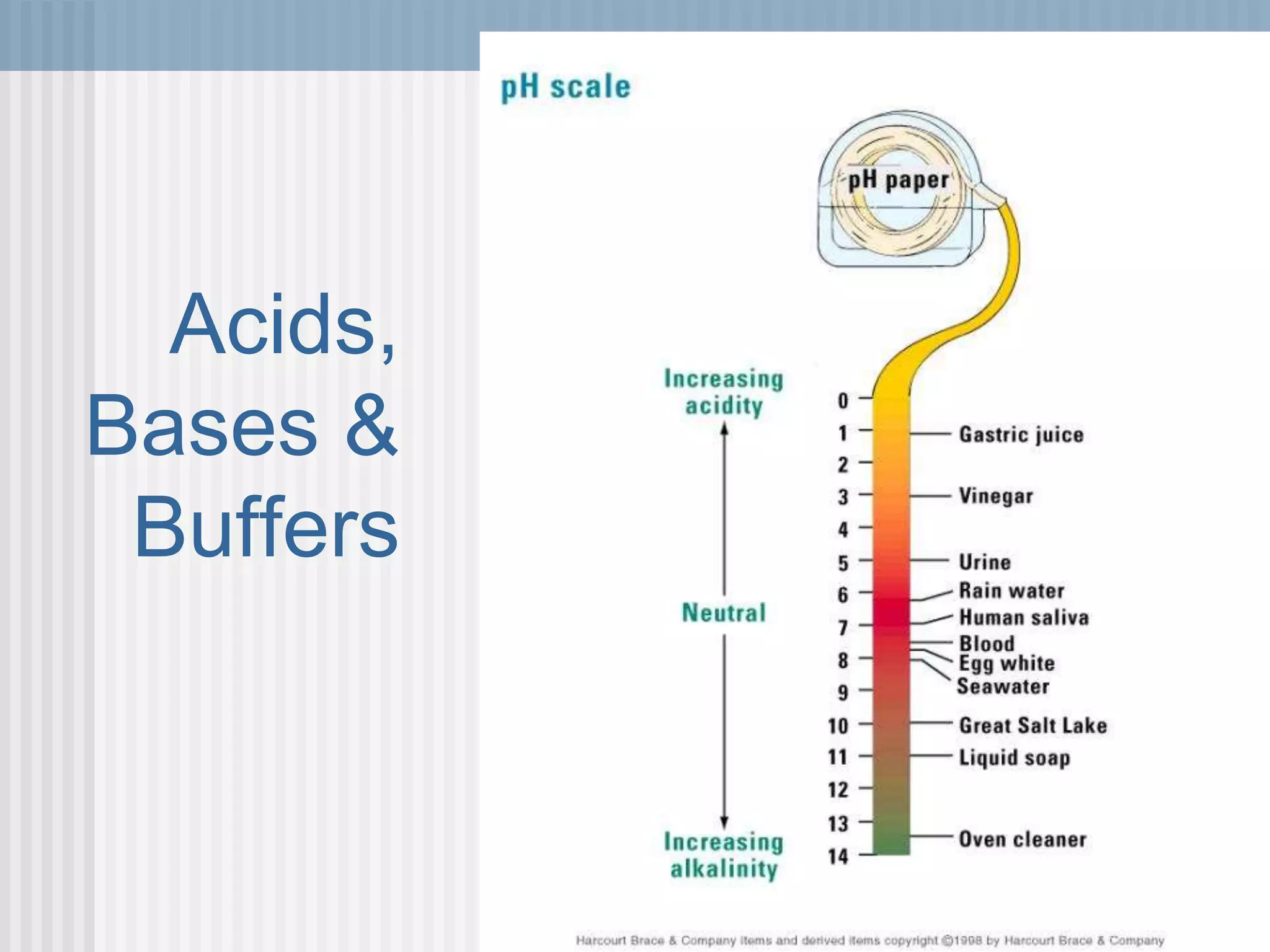
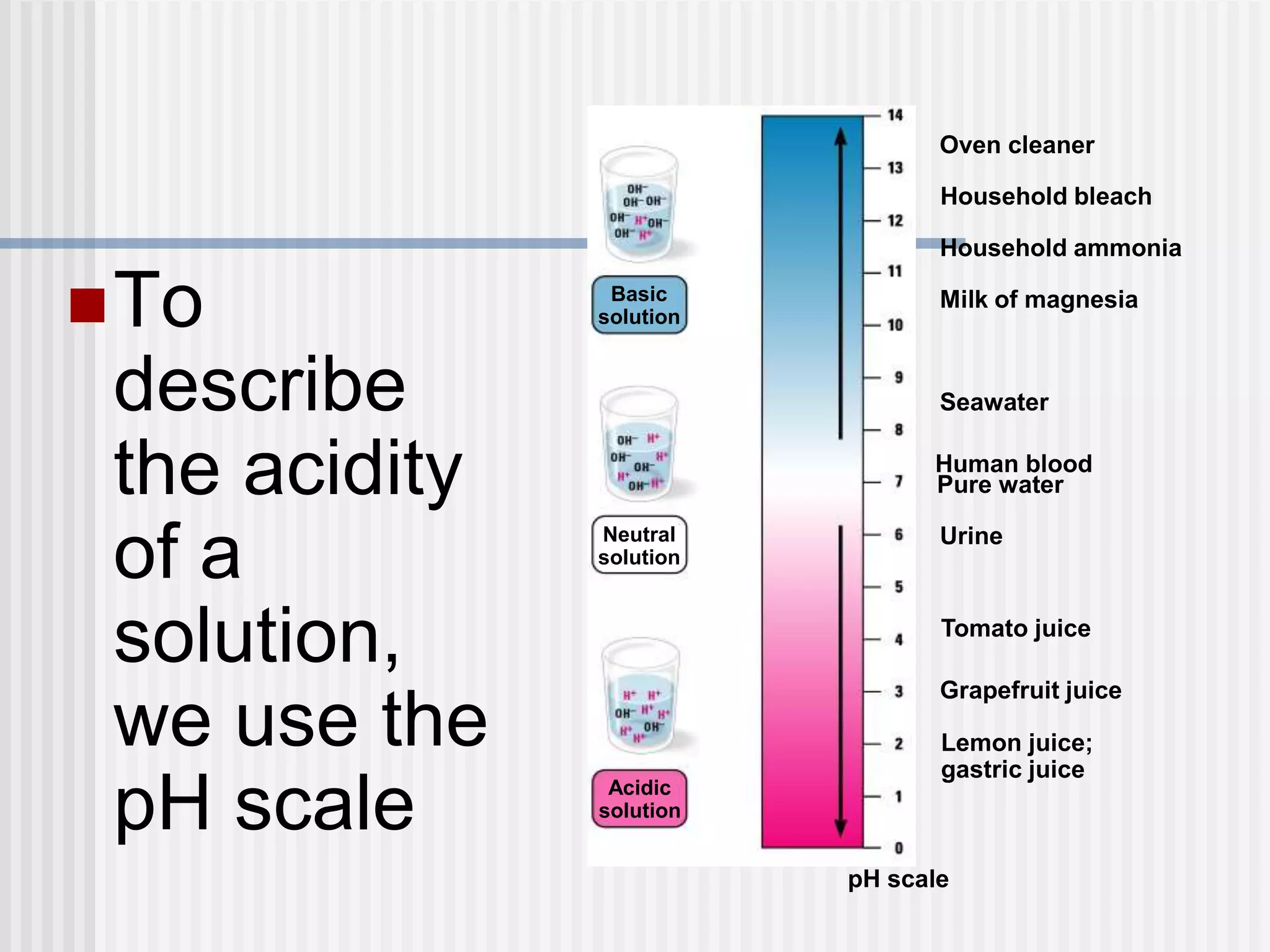
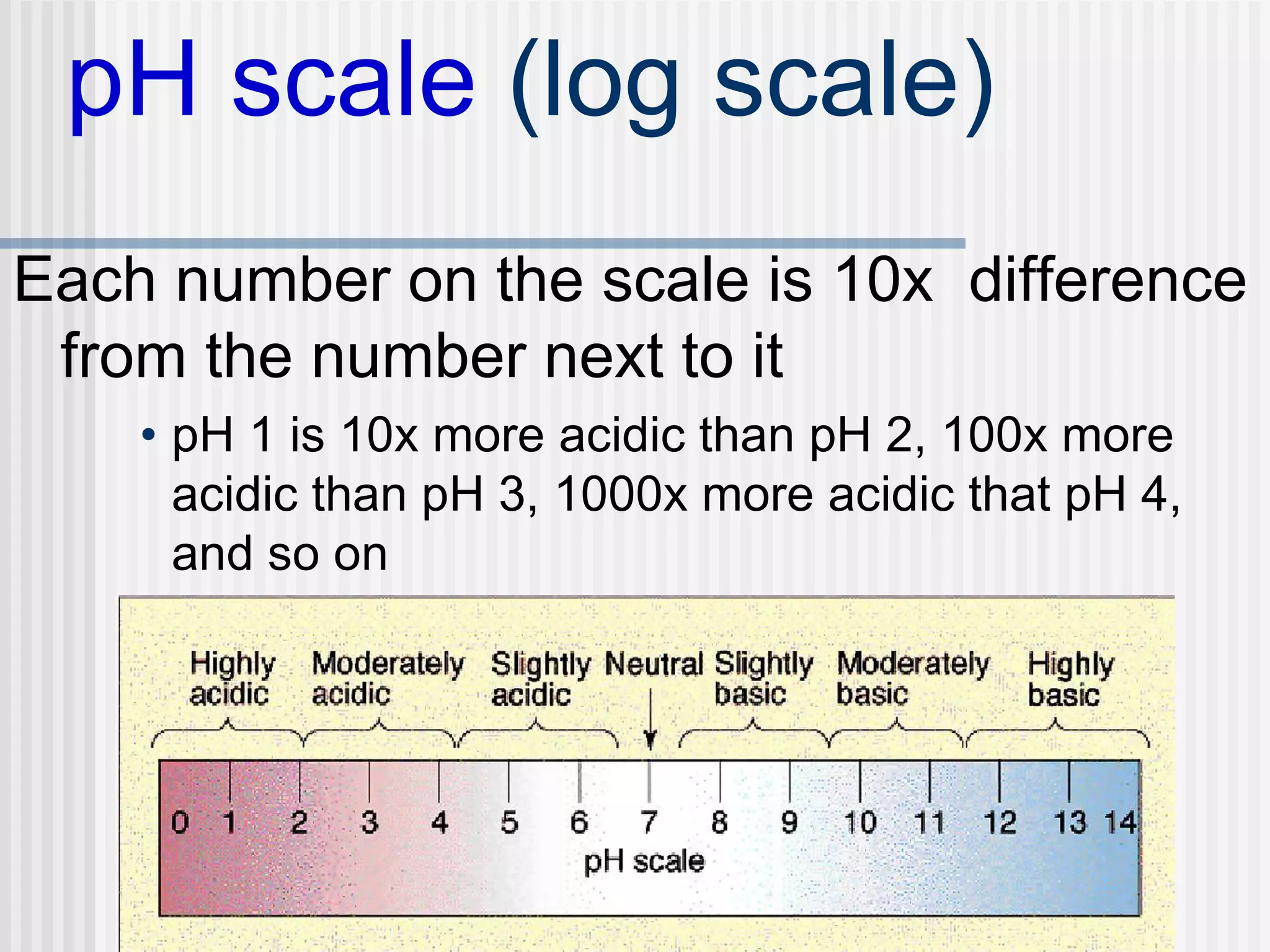
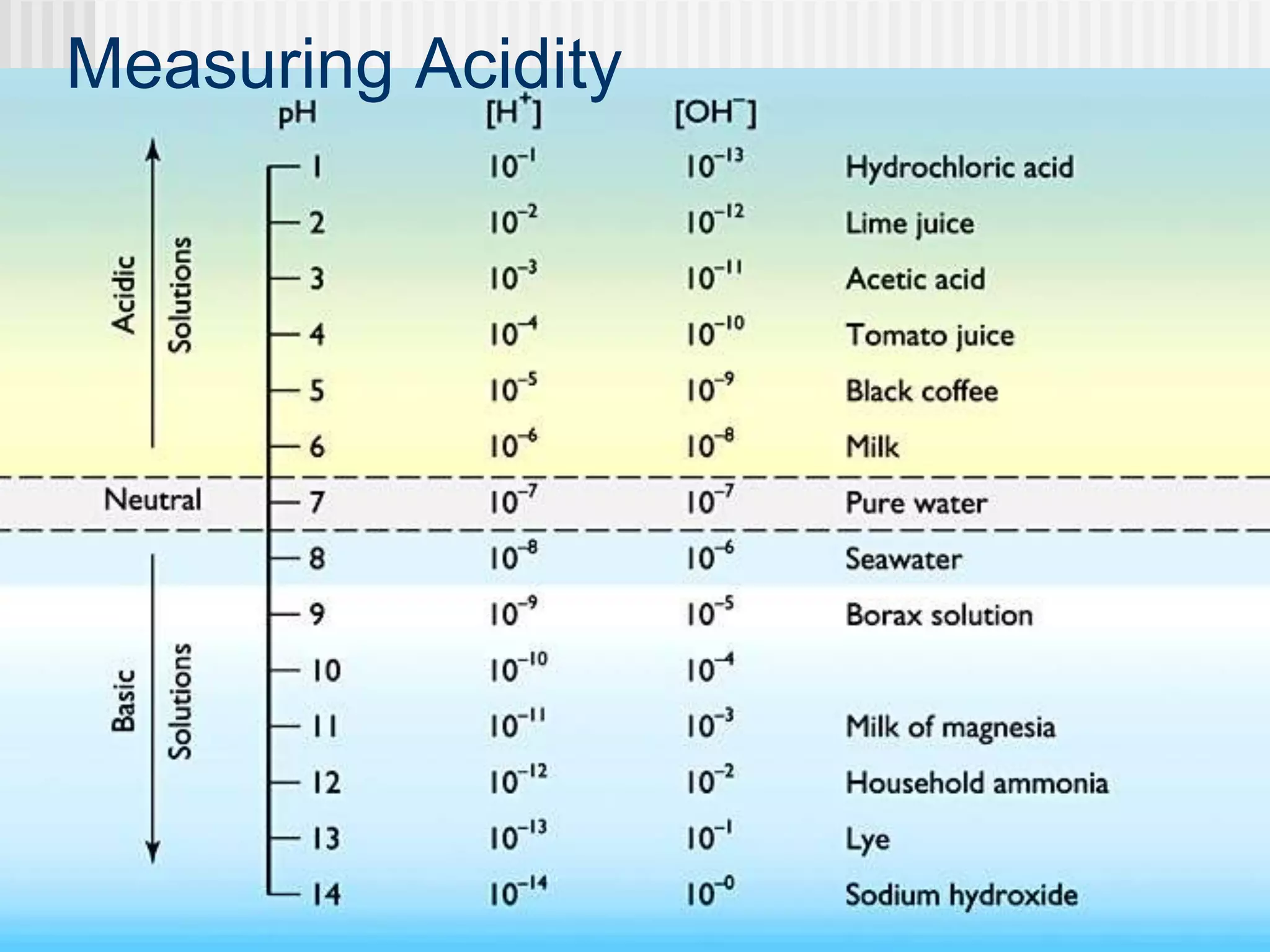
The document discusses several key properties of water that support life. It explains that water is polar due to oxygen's electronegativity pulling the electrons towards it in the water molecule. This polarity allows hydrogen bonds to form between water molecules, giving water properties like high surface tension and ability to moderate temperature that help support life. The document also discusses how water's polarity allows it to be an excellent solvent and how buffers help regulate pH.