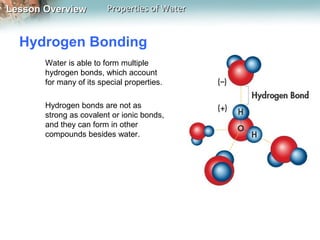
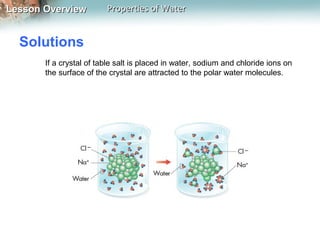
This document discusses the unique properties of water that make it essential for life. Water is a polar molecule that can form hydrogen bonds between oxygen and hydrogen atoms of neighboring water molecules. This allows water to have high cohesion and surface tension as well as be an excellent solvent. Water's polarity and hydrogen bonding give it a high heat capacity, allowing it to absorb large amounts of heat with minimal temperature changes. These properties are critical for living organisms and allow water to act as a universal solvent for biological molecules and reactions.