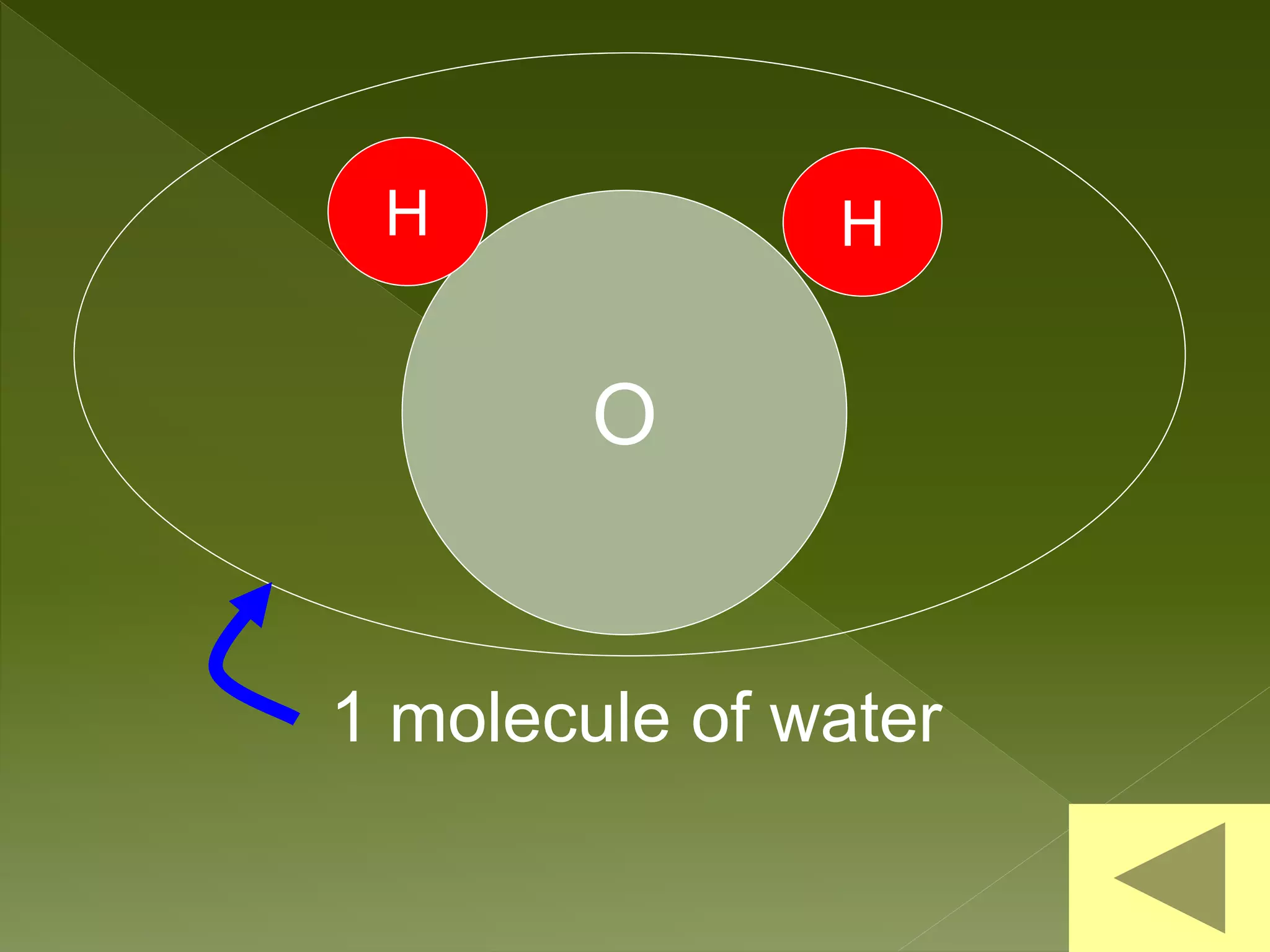
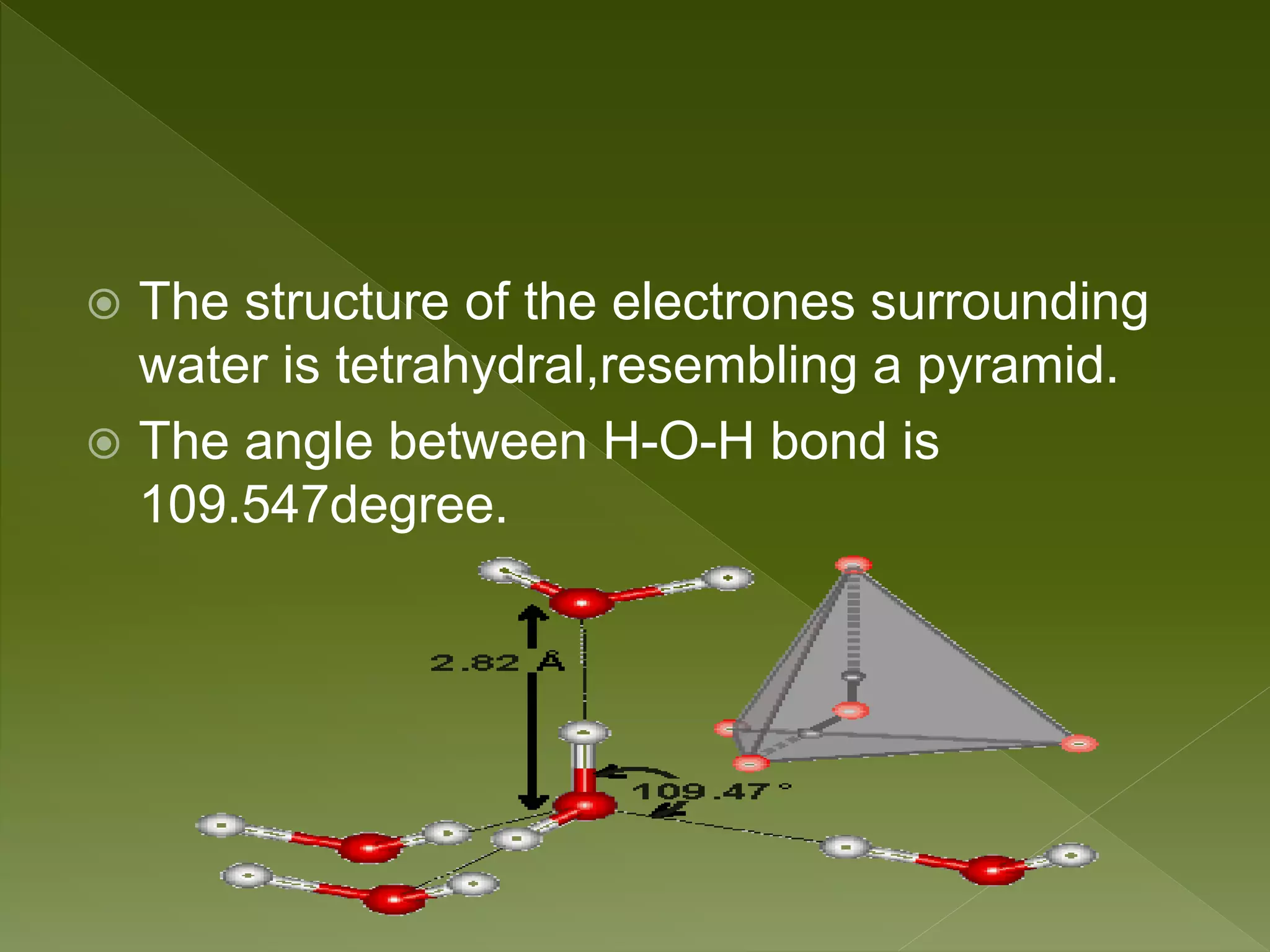
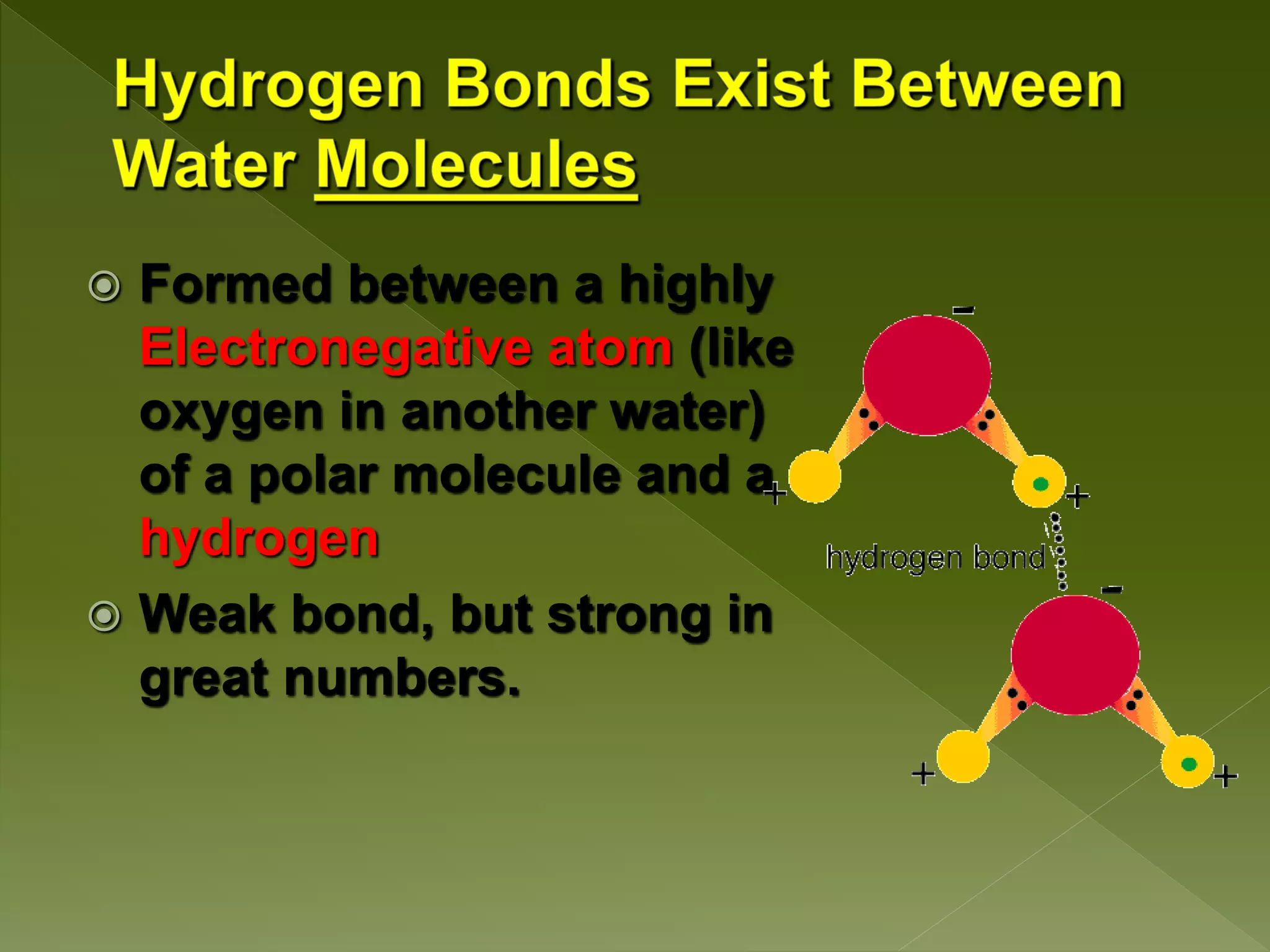
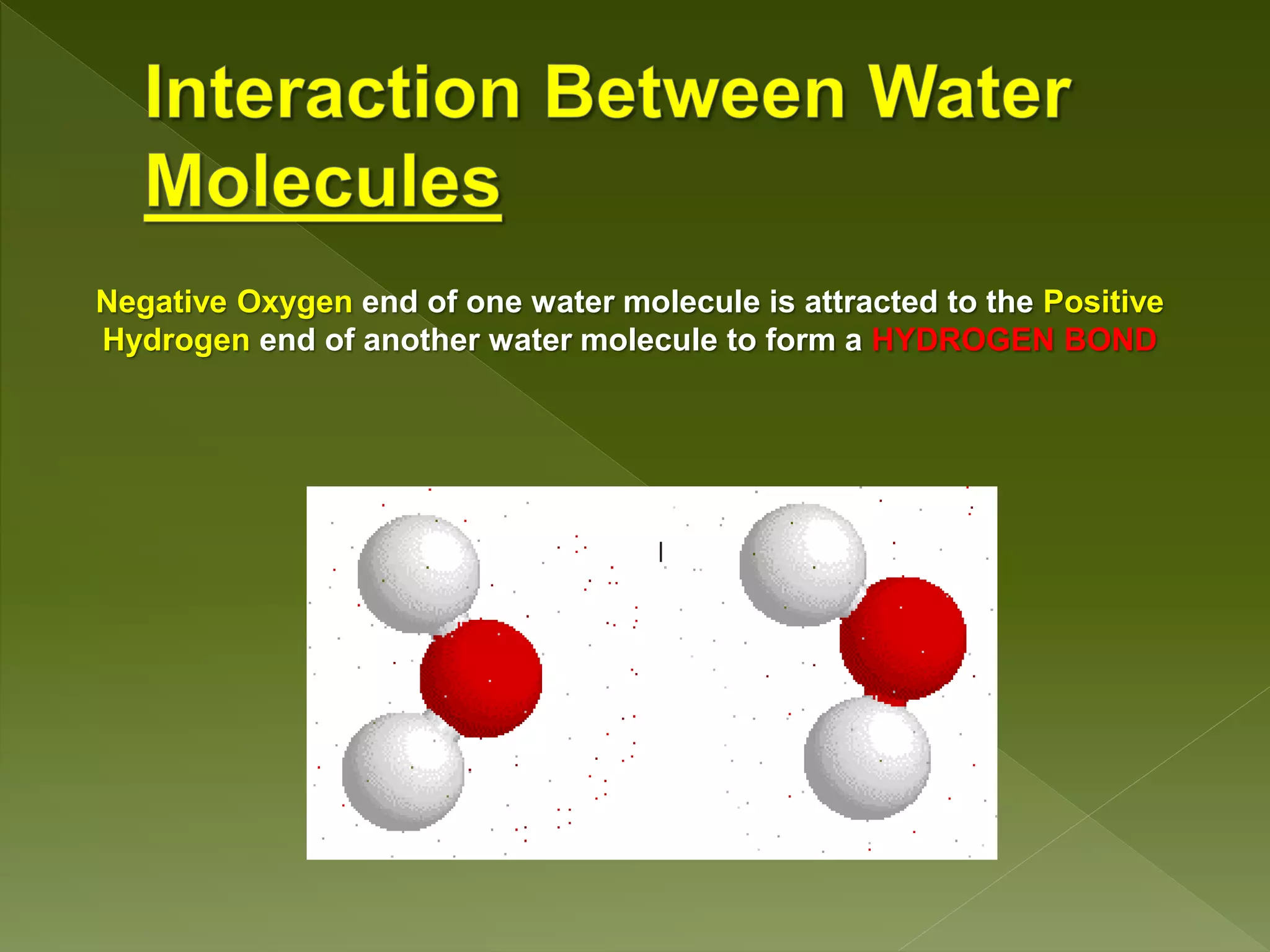
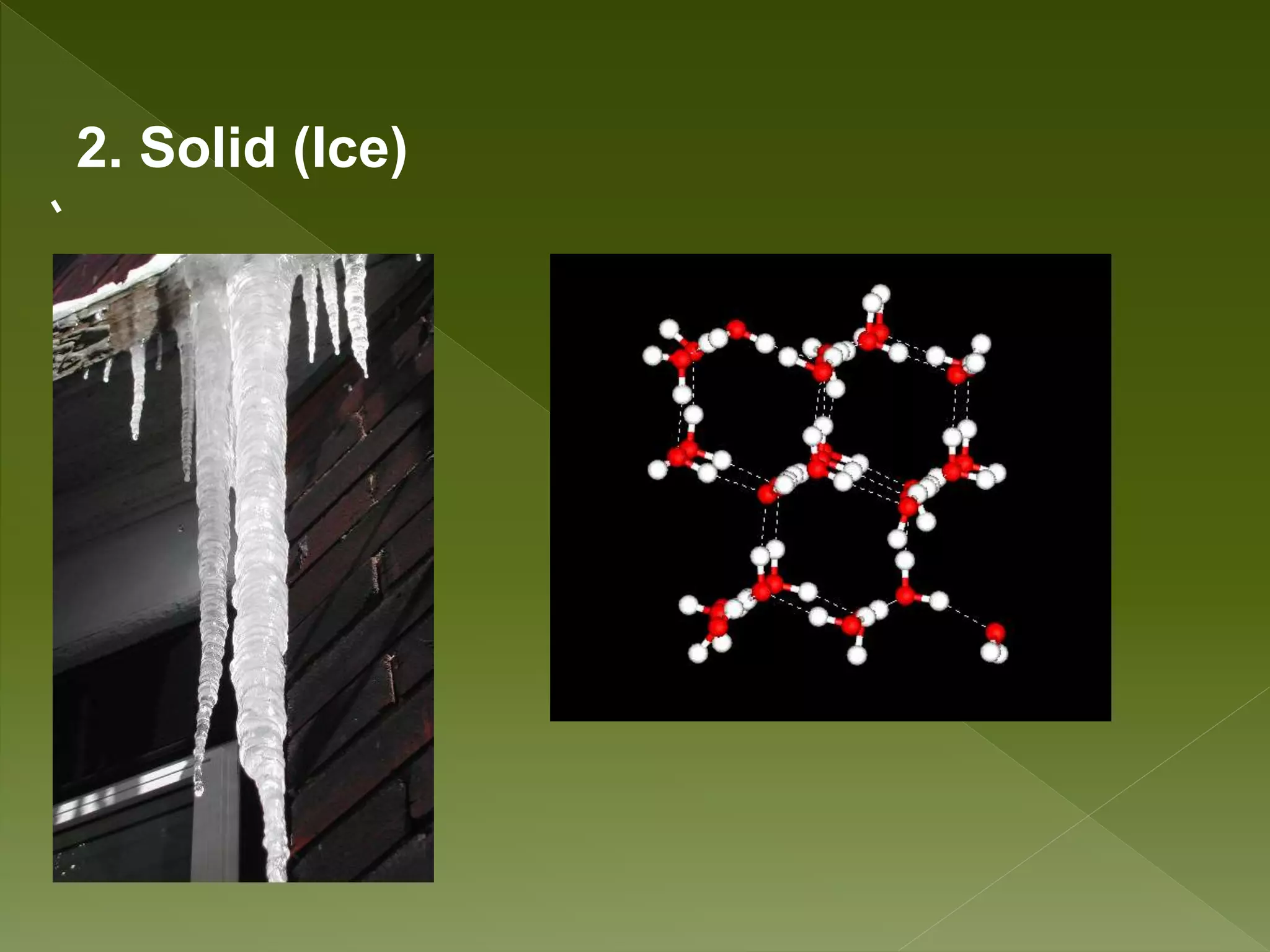
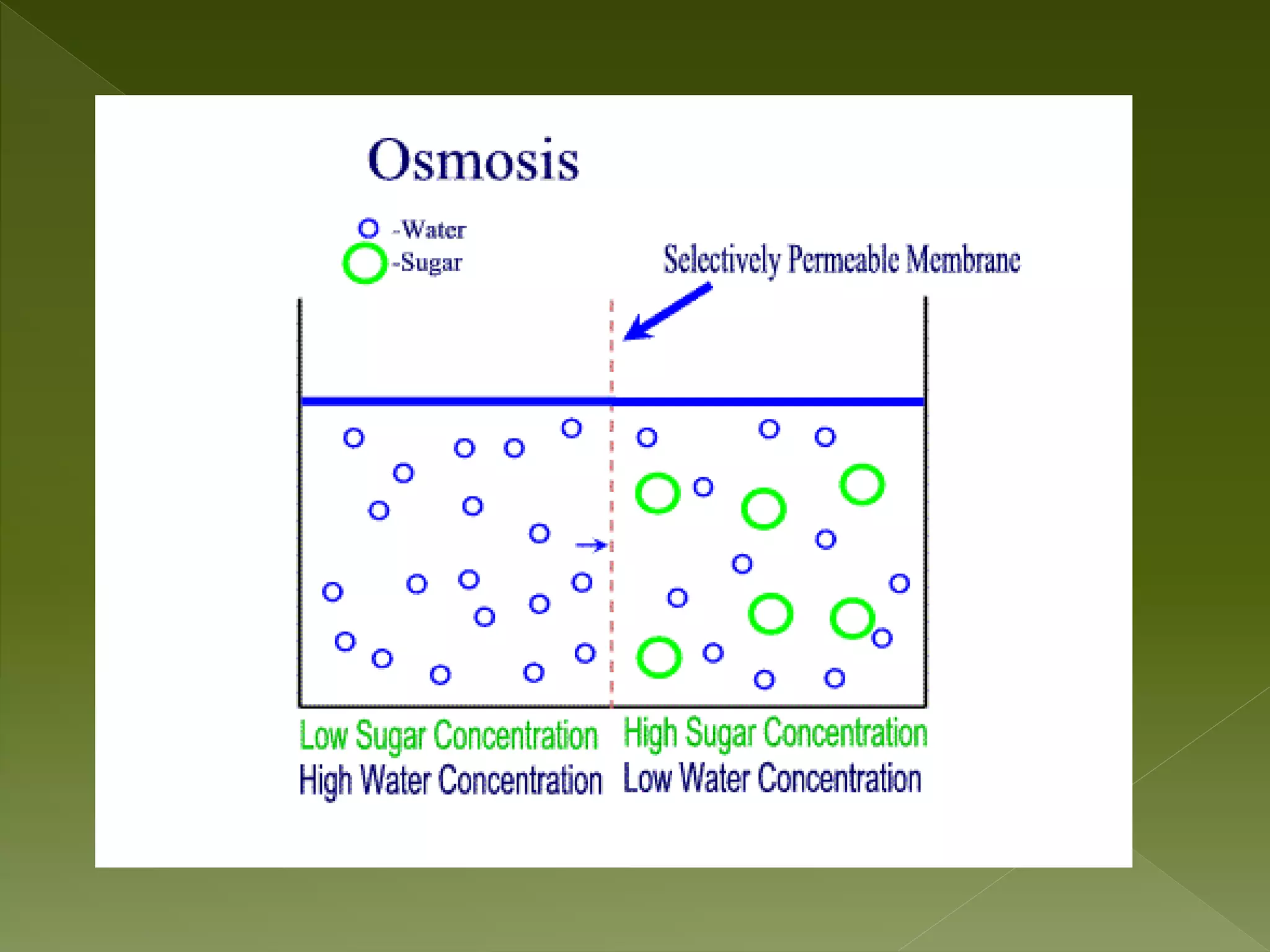
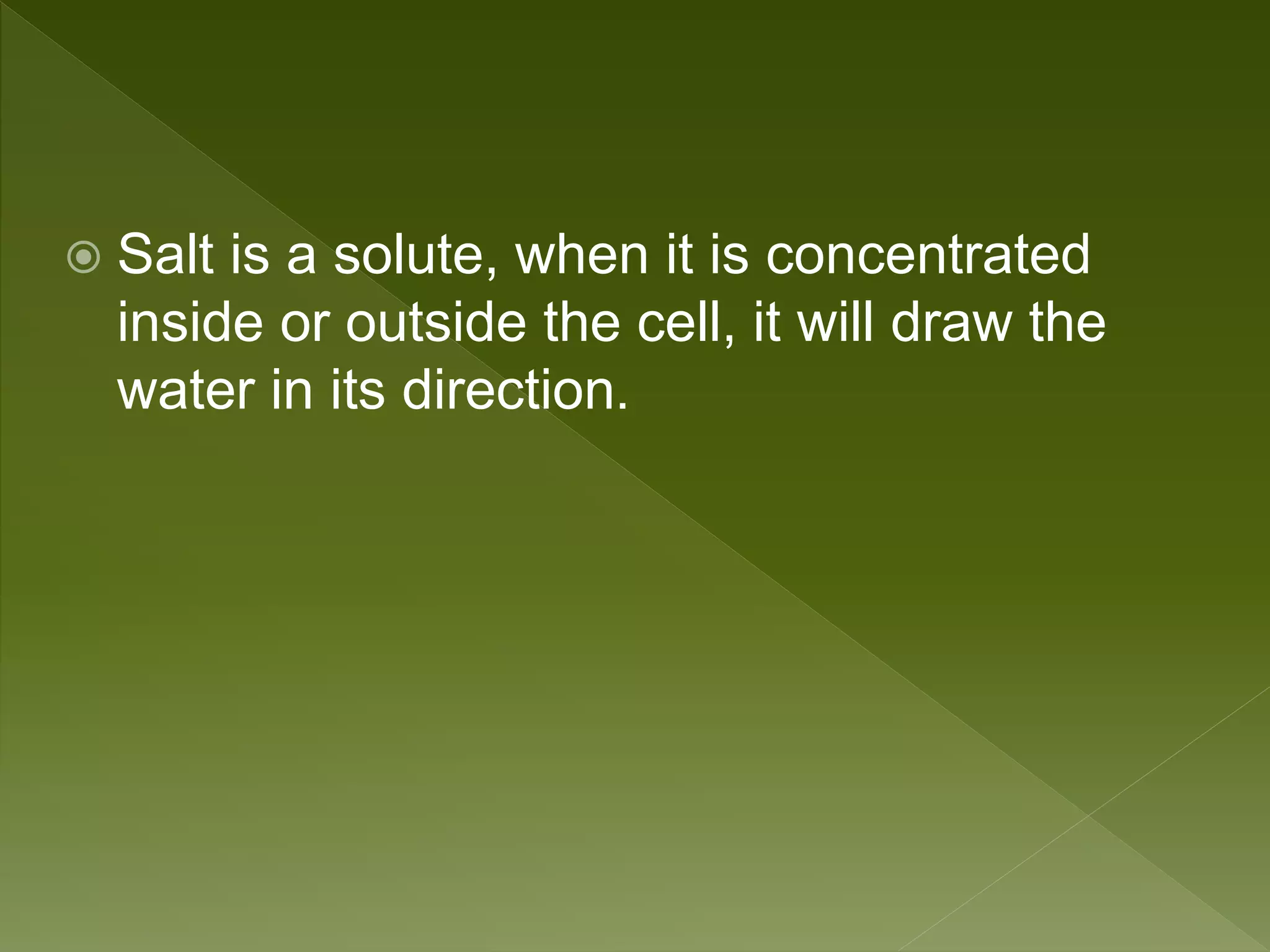
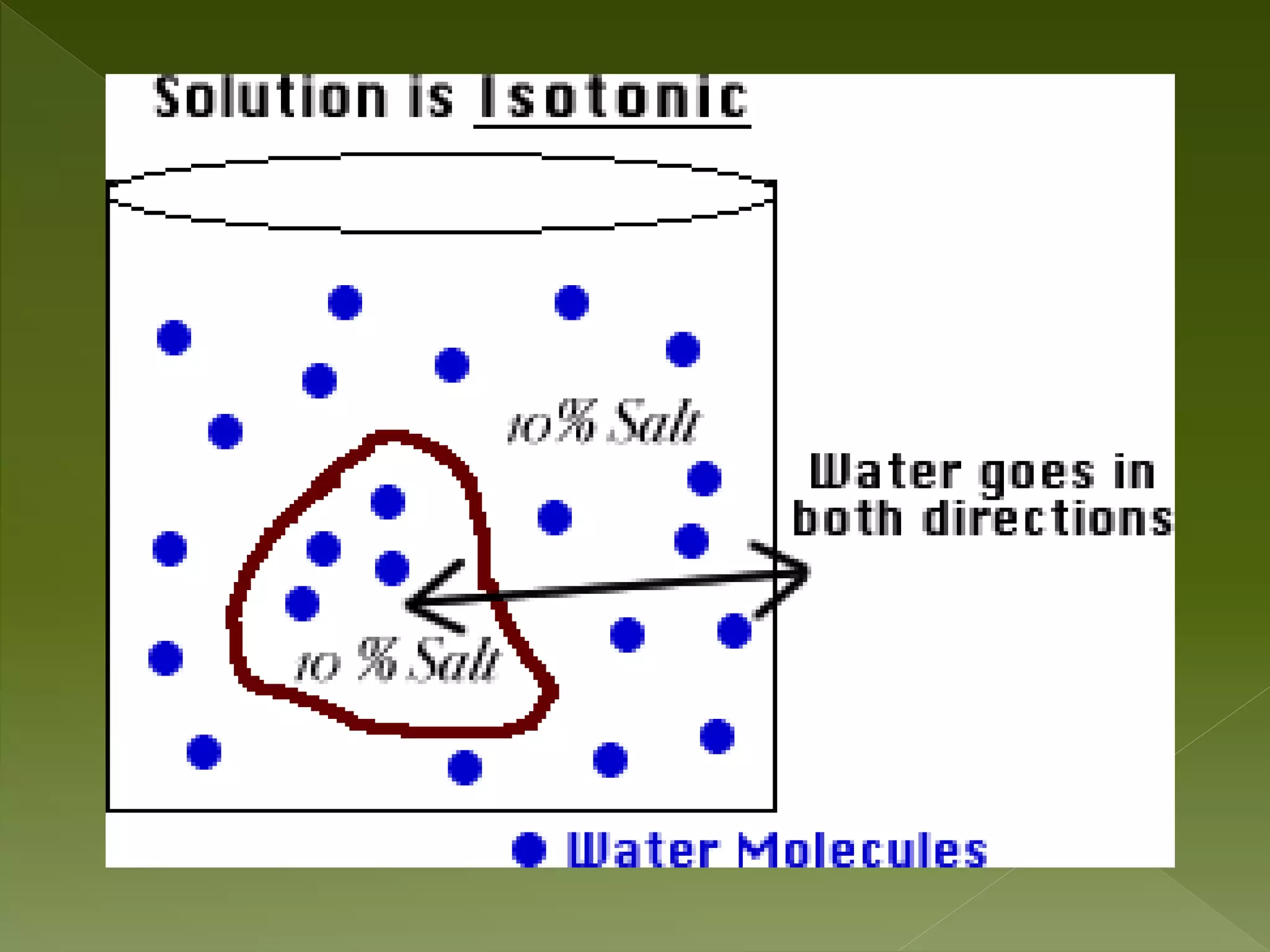
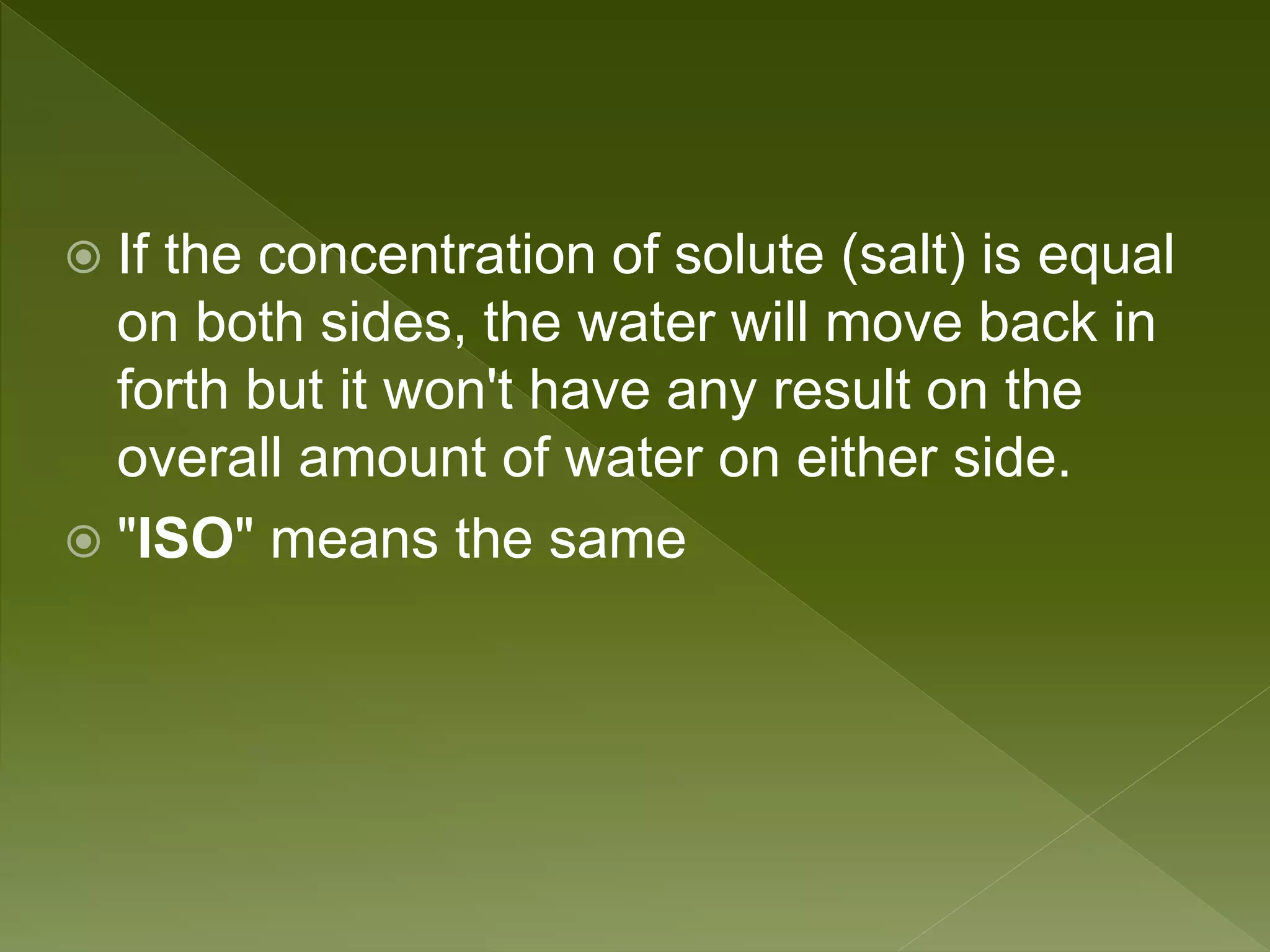
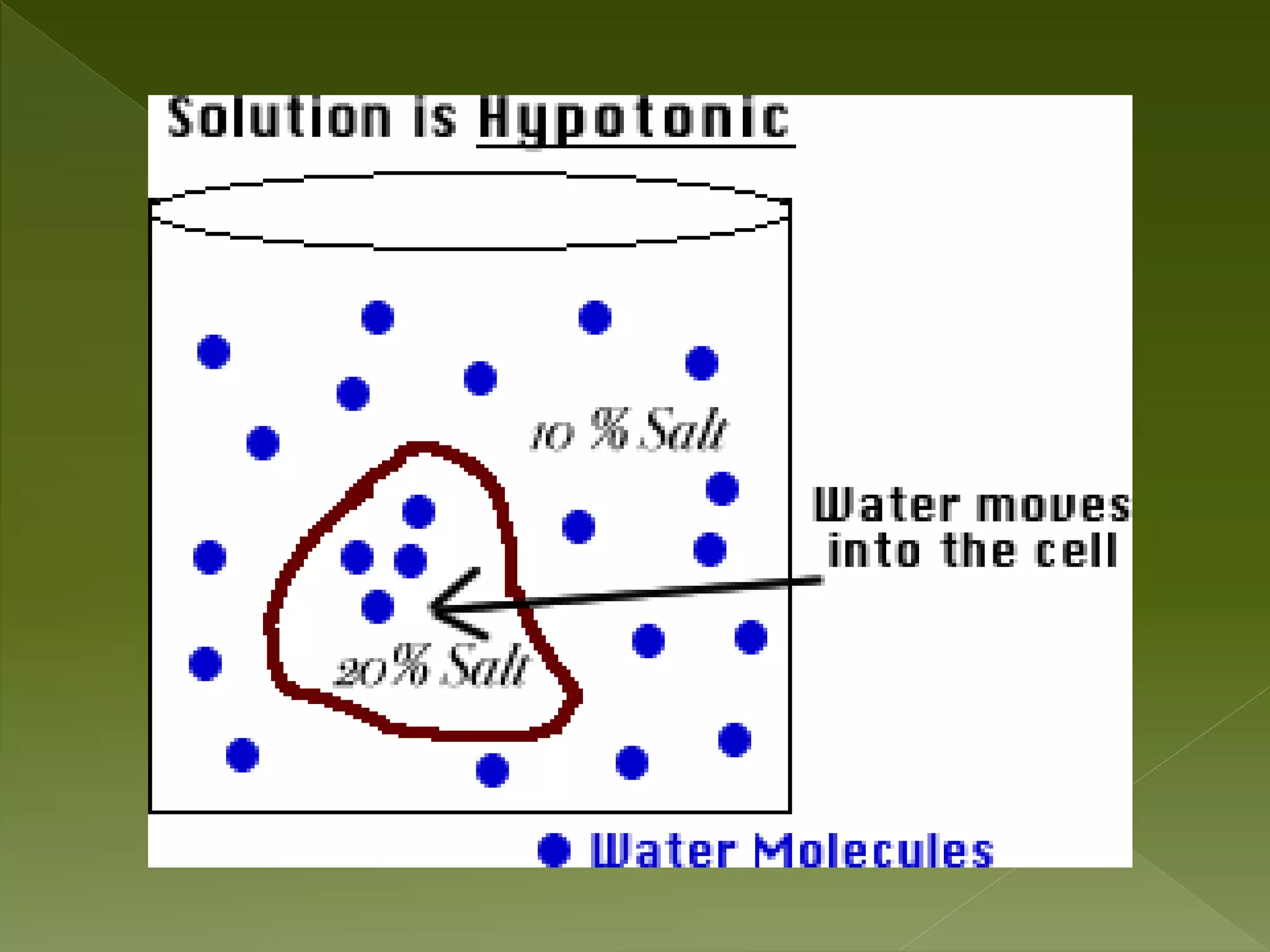
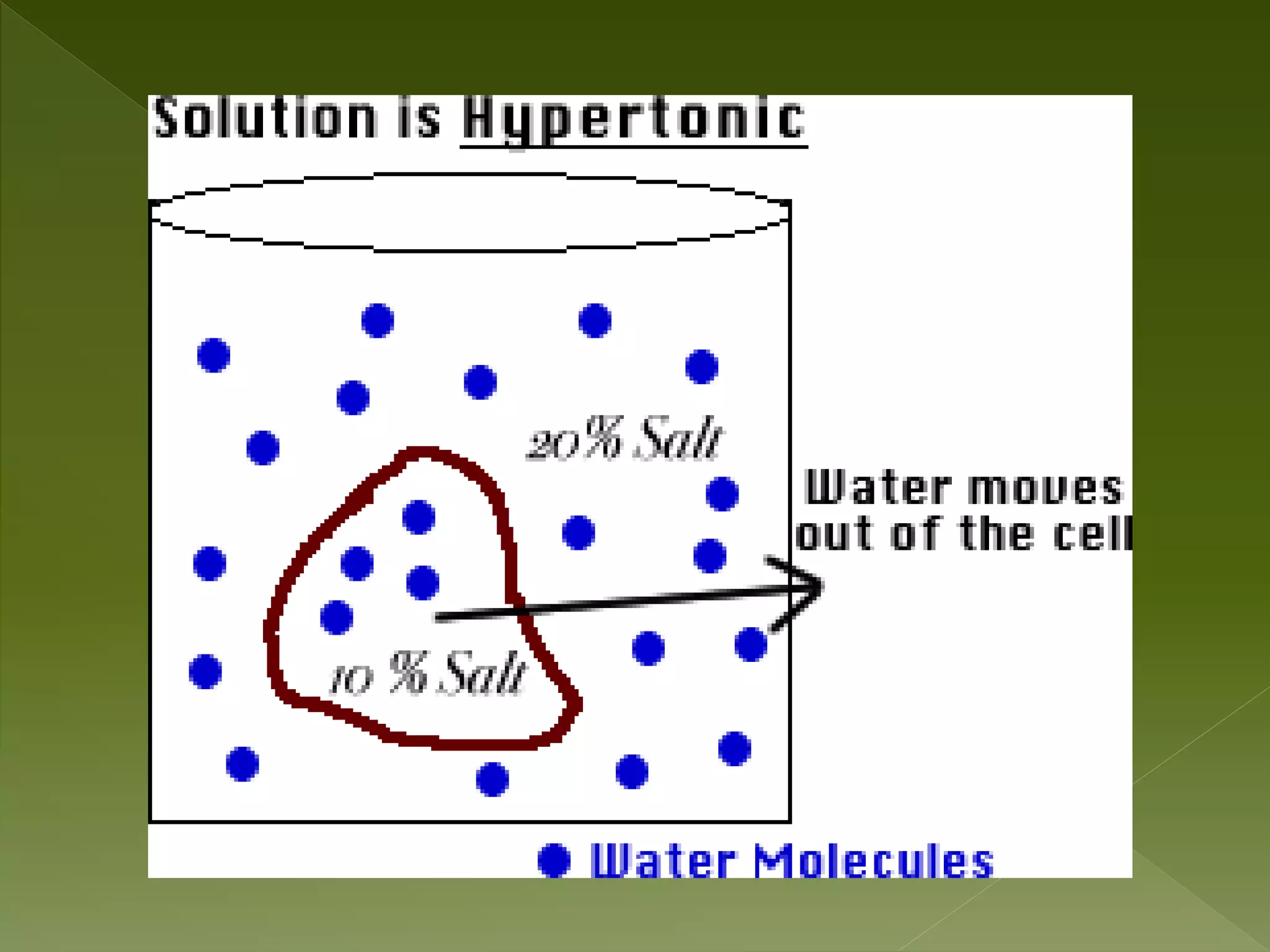
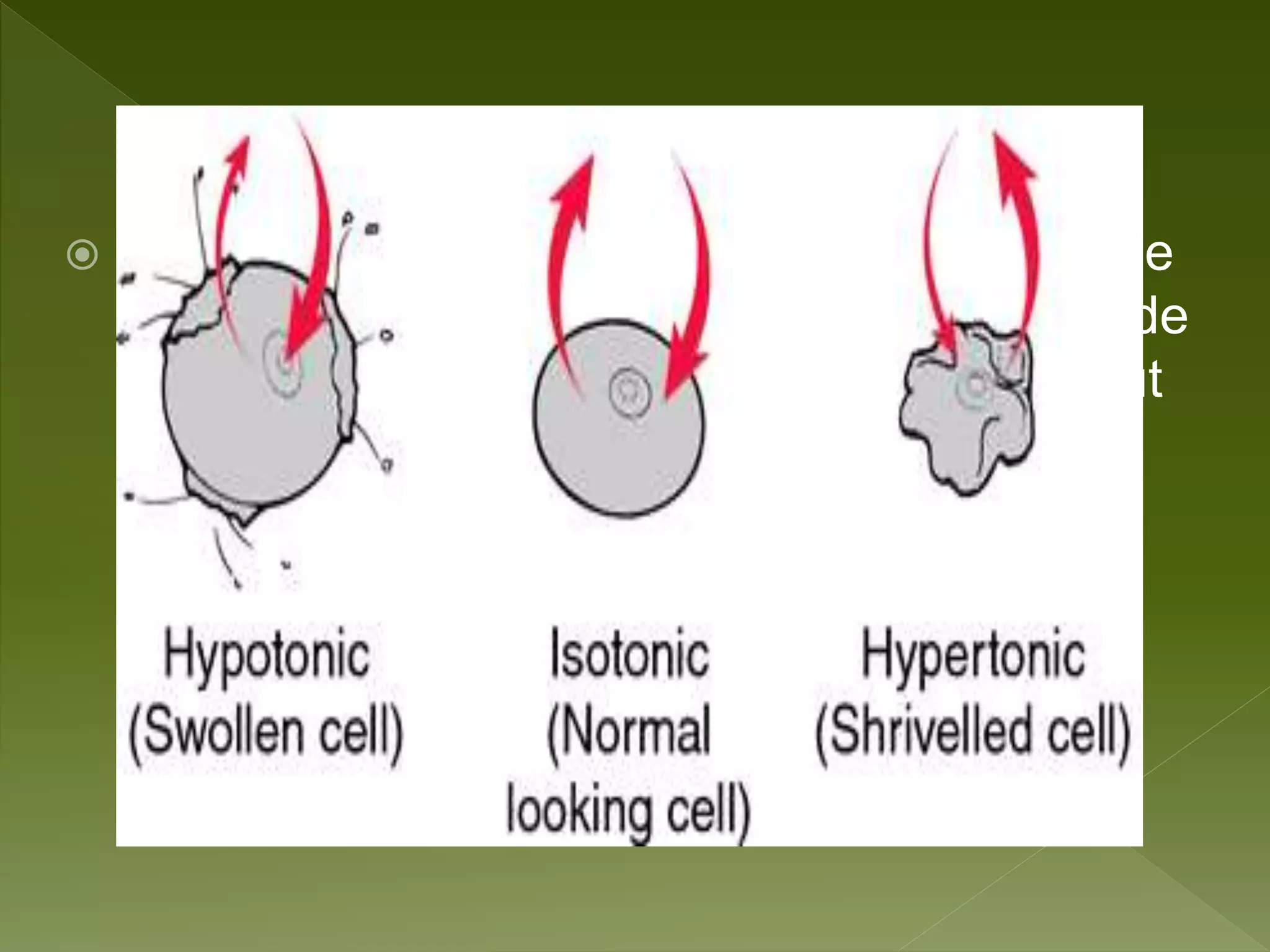
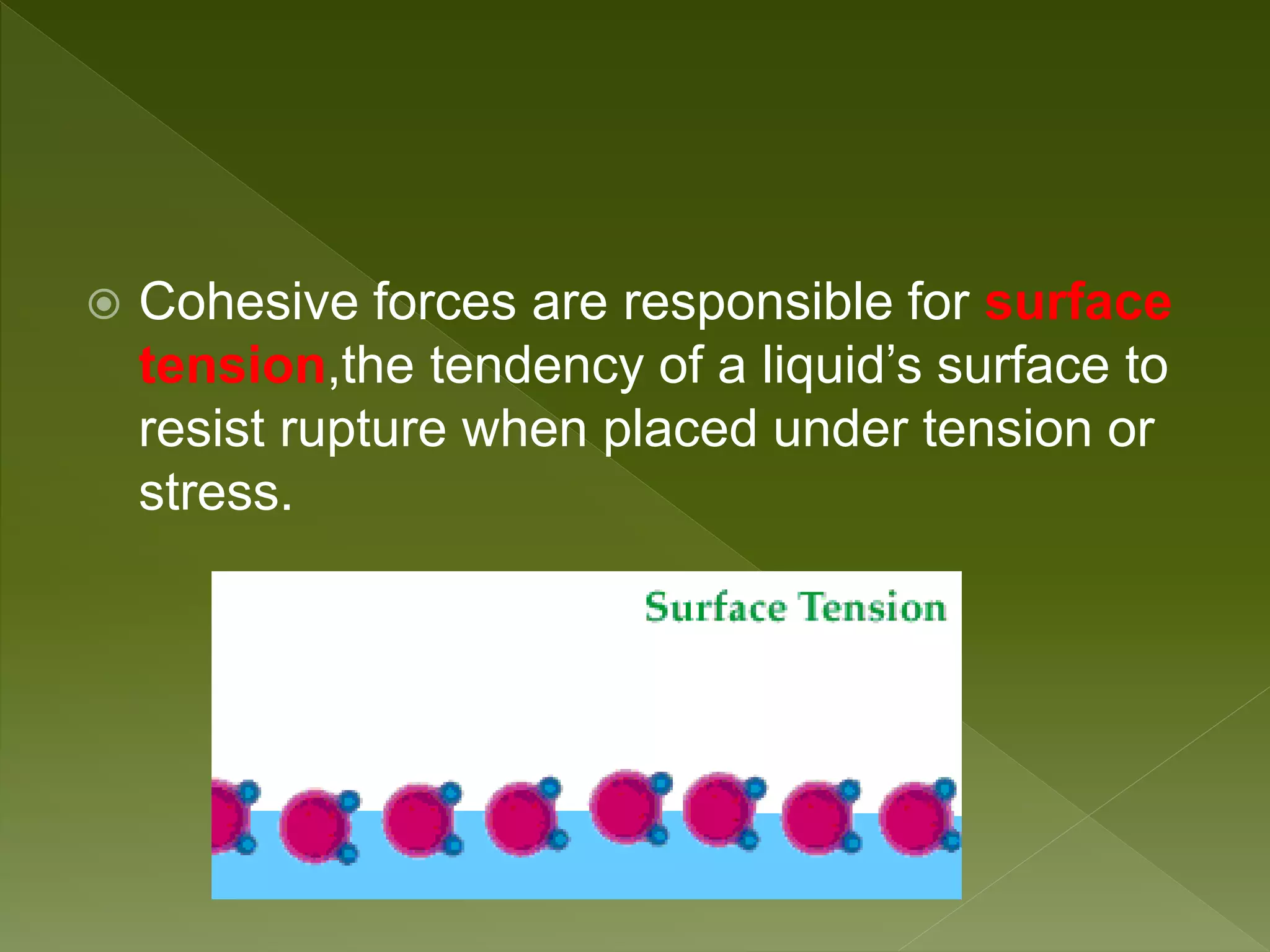
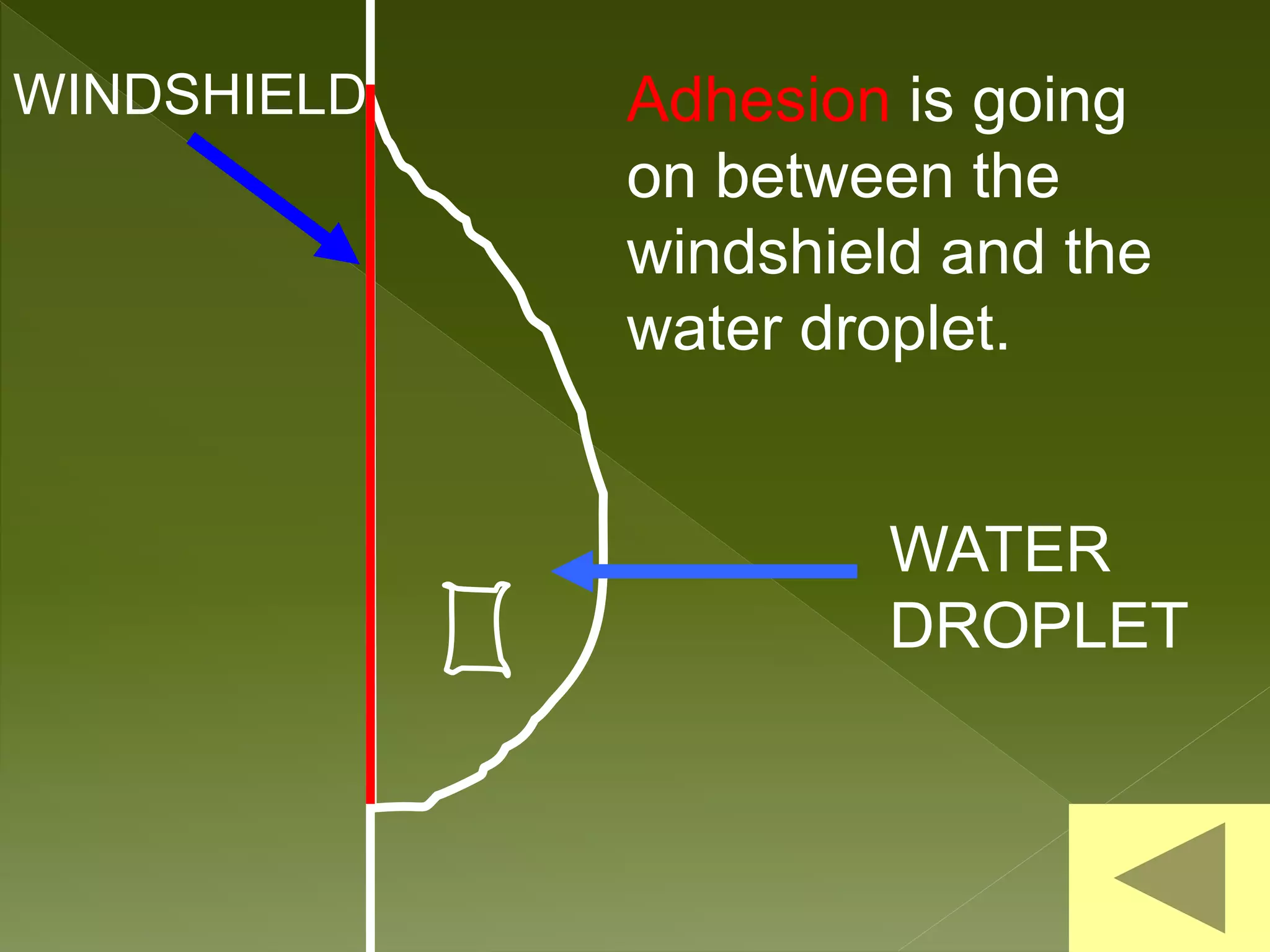
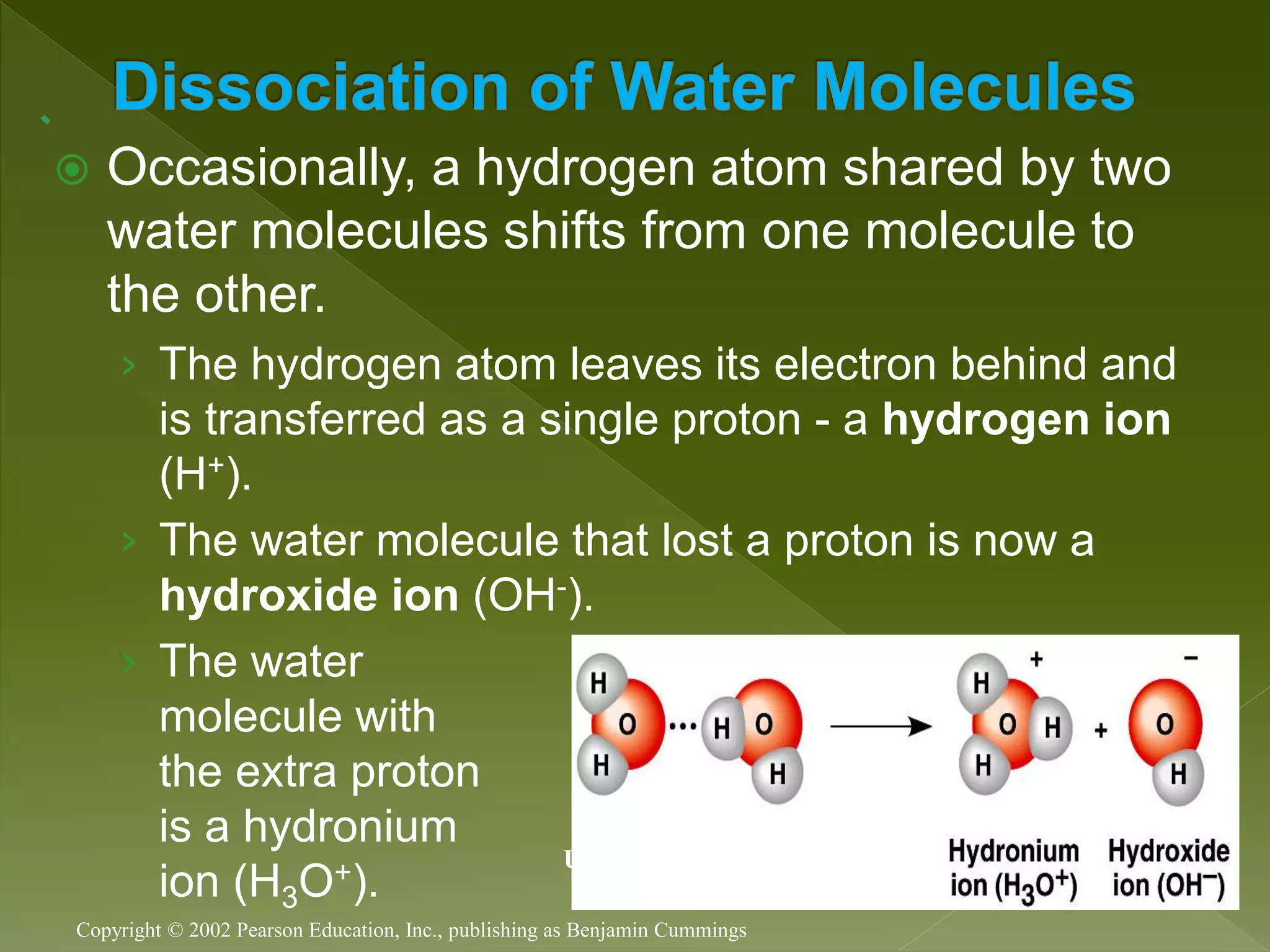
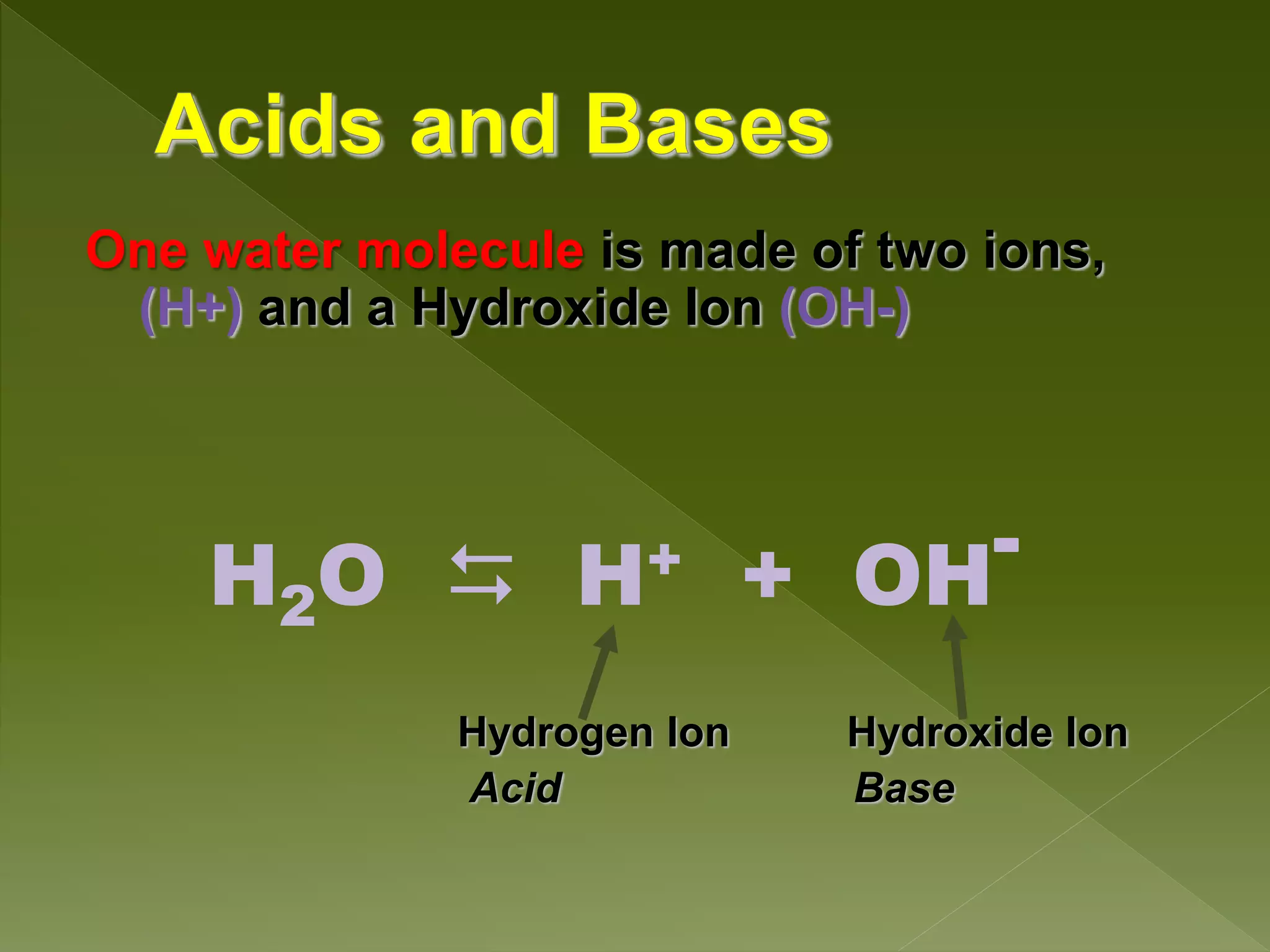
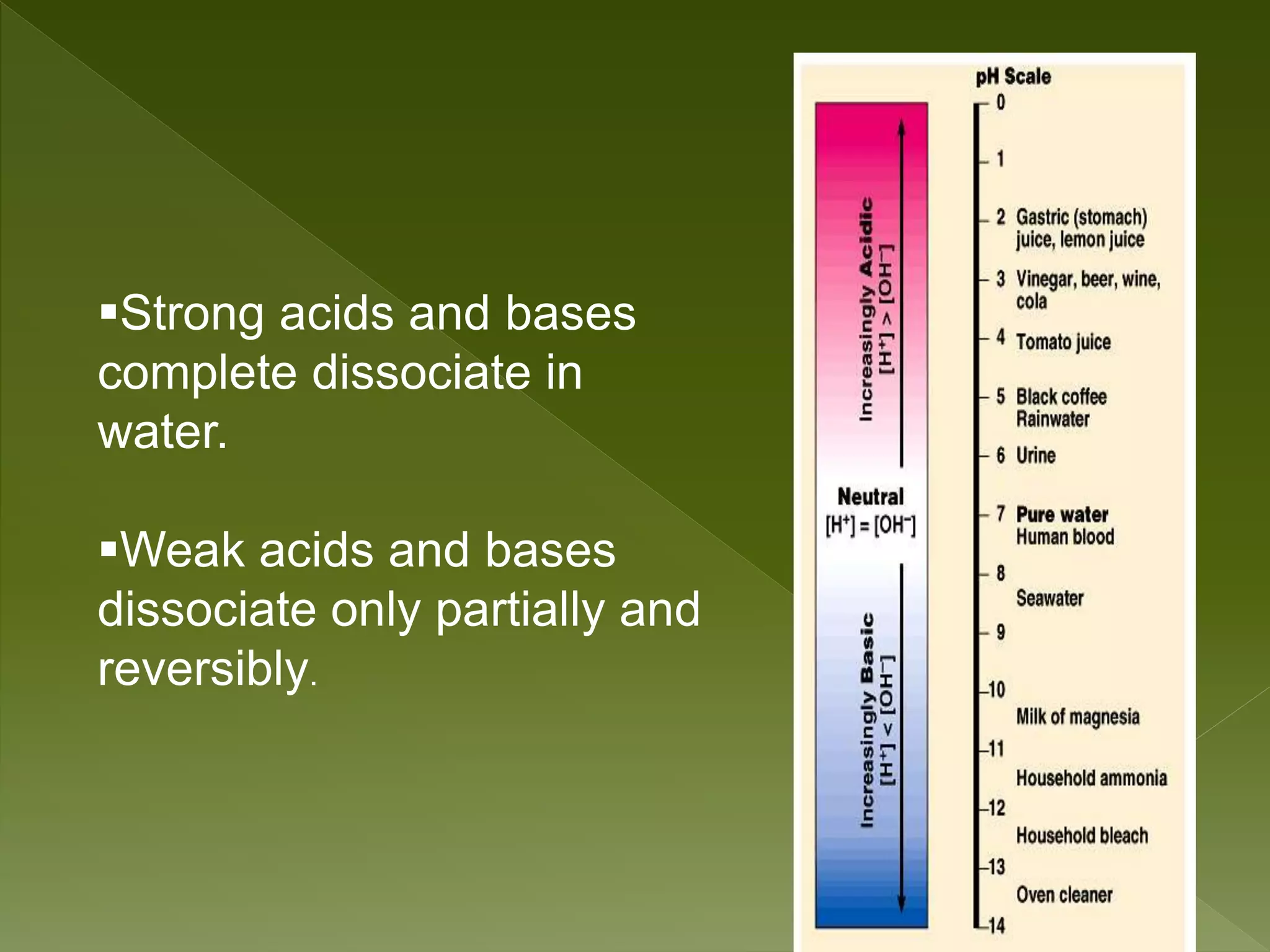
Water is a polar molecule comprised of an oxygen atom covalently bonded to two hydrogen atoms at an angle of 104.5 degrees. This polar structure results in slightly positive and negative charges on opposite sides of the molecule. Water molecules can form hydrogen bonds between the slightly positive hydrogen of one molecule and the slightly negative oxygen of another. These hydrogen bonds give water important properties like surface tension, capillary action, and allowing it to dissolve many other polar substances. Water can also exist as a liquid, solid (ice), and gas (water vapor) and plays a vital role in all living things.