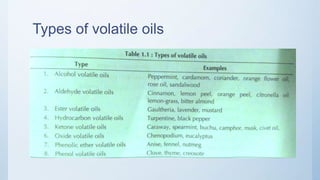
The document discusses volatile oils, also known as essential oils, which are characterized by their odorous, evaporative principles derived from plants and animals. It covers the extraction processes, types, chemical tests, storage, and various uses of volatile oils in pharmaceuticals, food, cosmetics, and medicine. Additionally, it highlights terpeneless oils that are valued in the perfumery industry for their stability and specificity.