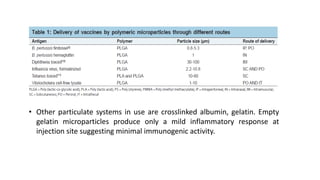
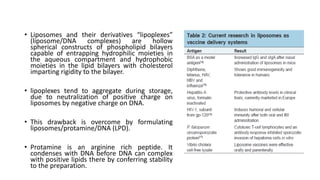
This document discusses various vaccine delivery systems including particulate systems, liposomal systems, virosomes, emulsion systems, polymeric nanoparticles, micellar systems, dendrimer-based systems, immunostimulatory complexes (ISCOMs), and DNA vaccines. It provides details on the composition and mechanisms of these different delivery systems and how they can be used to deliver vaccine antigens and induce protective immunity. Physical and mucosal delivery methods are also summarized for enhancing DNA vaccine delivery.