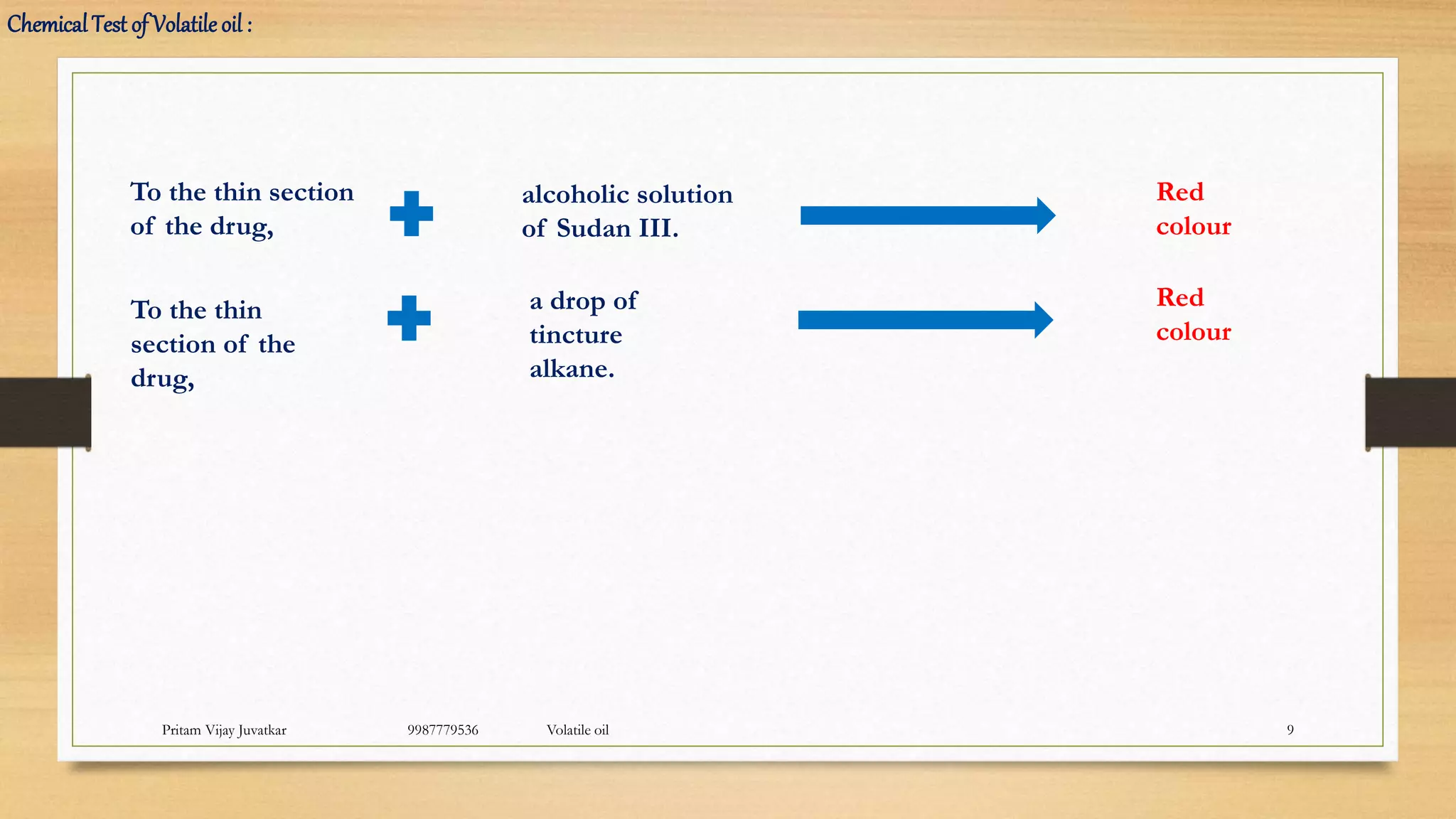
The document provides an overview of volatile oils, also known as ethereal or essential oils, including their definitions, classifications, properties, and extraction methods. It discusses various techniques such as hydrodistillation, enfleurage, and supercritical fluid extraction, as well as the different types of volatile oils categorized based on their chemical properties. Additionally, the document outlines the applications of these oils in pharmaceuticals, food, and cosmetics.