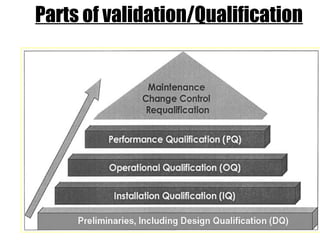
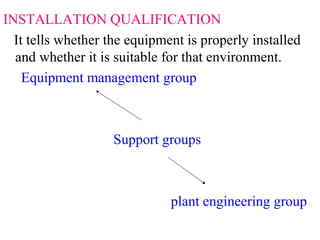
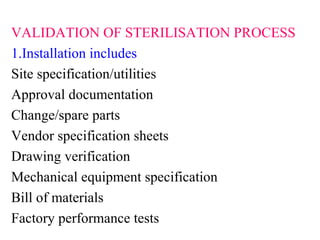
This document discusses validation of equipment used in pharmaceutical manufacturing. It defines validation and its objectives, which include improving reliability and safety. The main parts of validation are described as qualification including design, installation, operational, and performance qualification. Common equipment that undergo validation are listed, such as dissolution apparatus, autoclaves, and sterilization equipment. The roles of protocols, procedures, calibration, and regulatory agencies like the FDA in the validation process are also summarized.