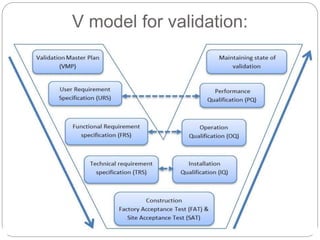
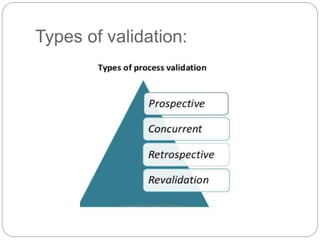
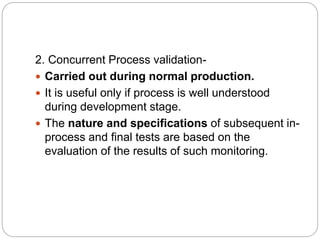
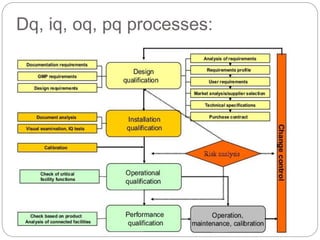
This document discusses validation, which is the process of establishing documented evidence that a system will consistently produce a product meeting its quality standards. It defines validation according to WHO and FDA and outlines the merits and scope of validation. It also discusses validation concepts like the validation master plan, V model, qualification processes for design, installation, operation and equipment, change control, and WHO guidelines for equipment validation. The types of validation covered are prospective, concurrent, retrospective and revalidation.