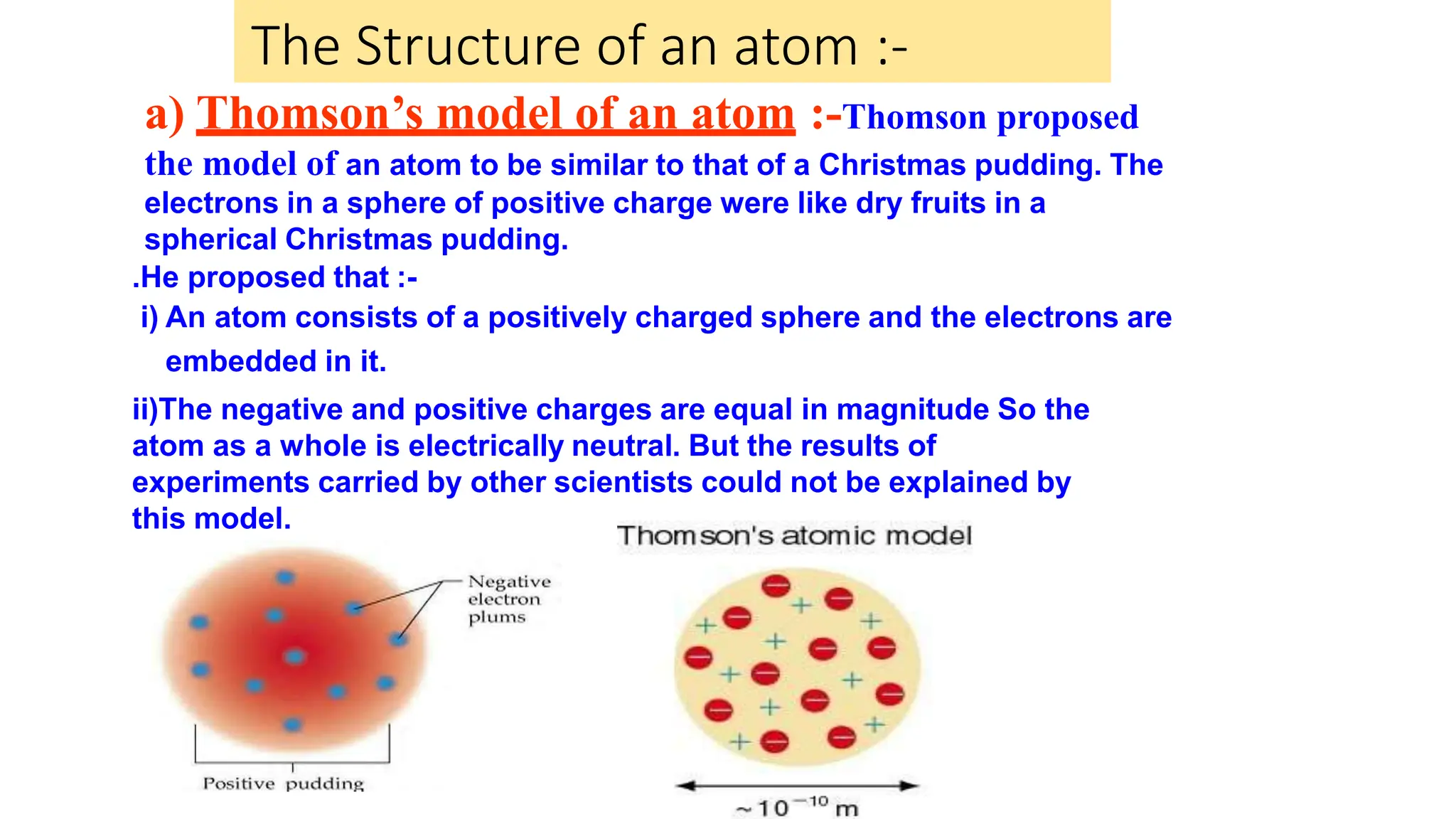
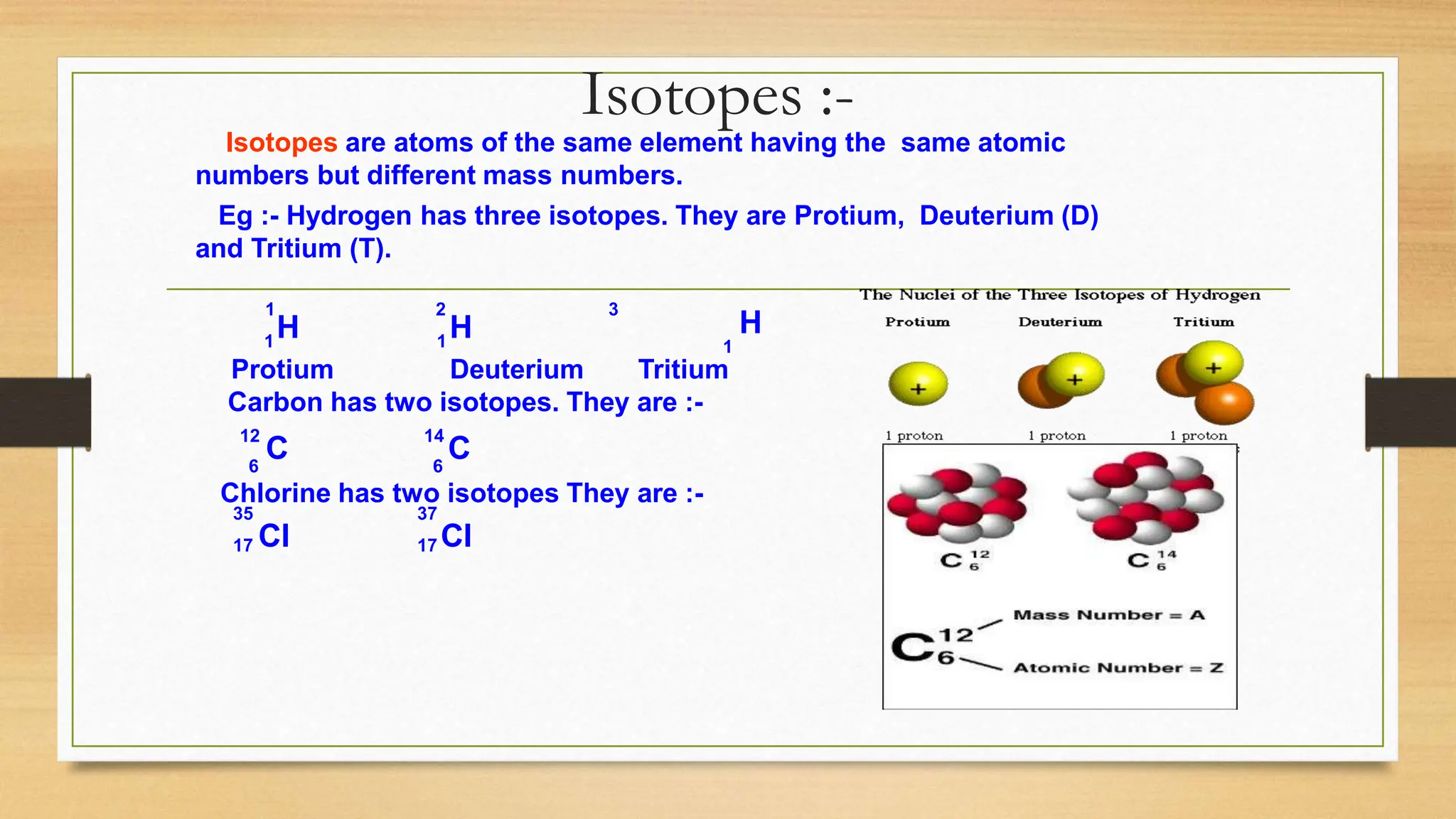
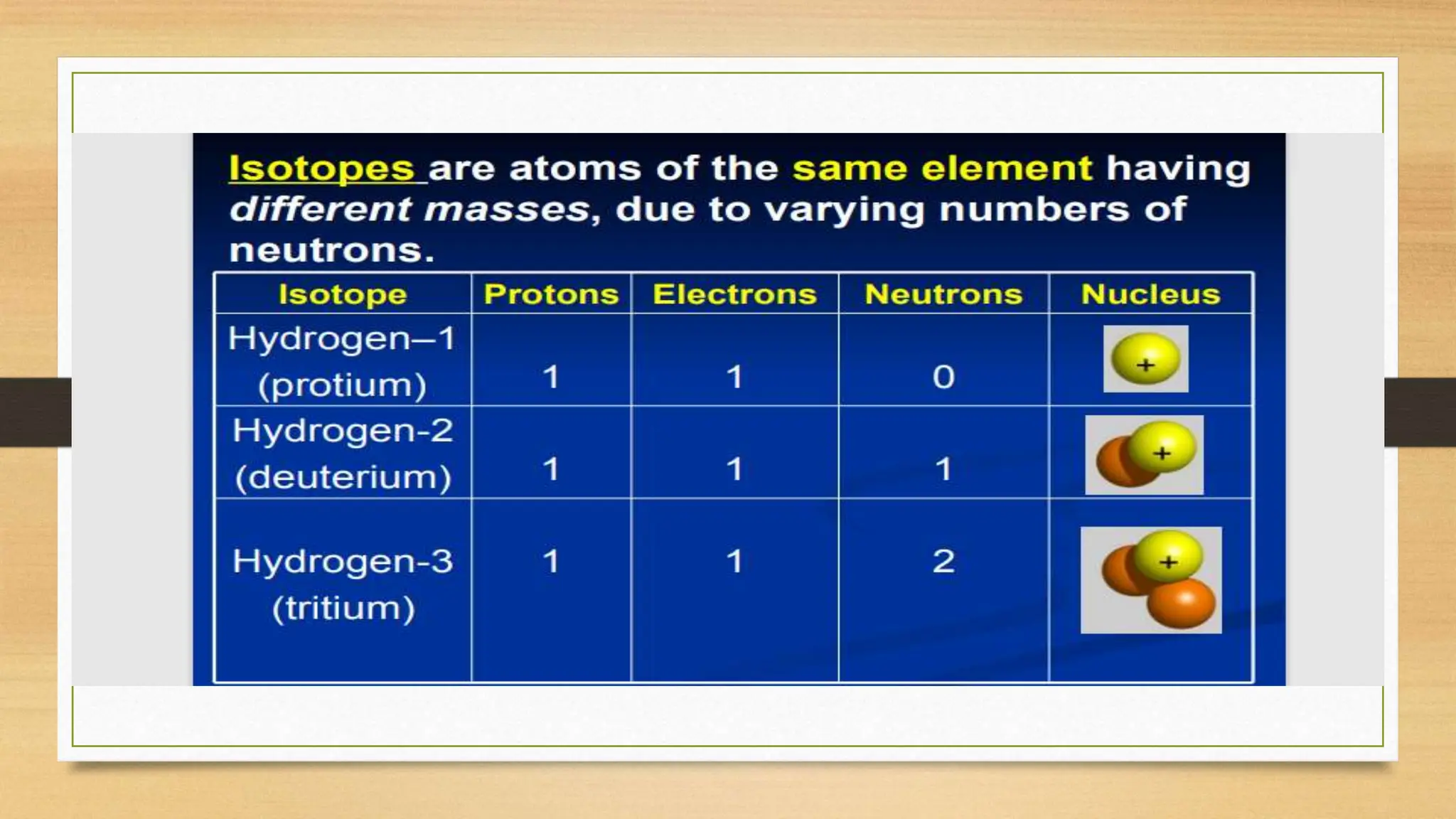
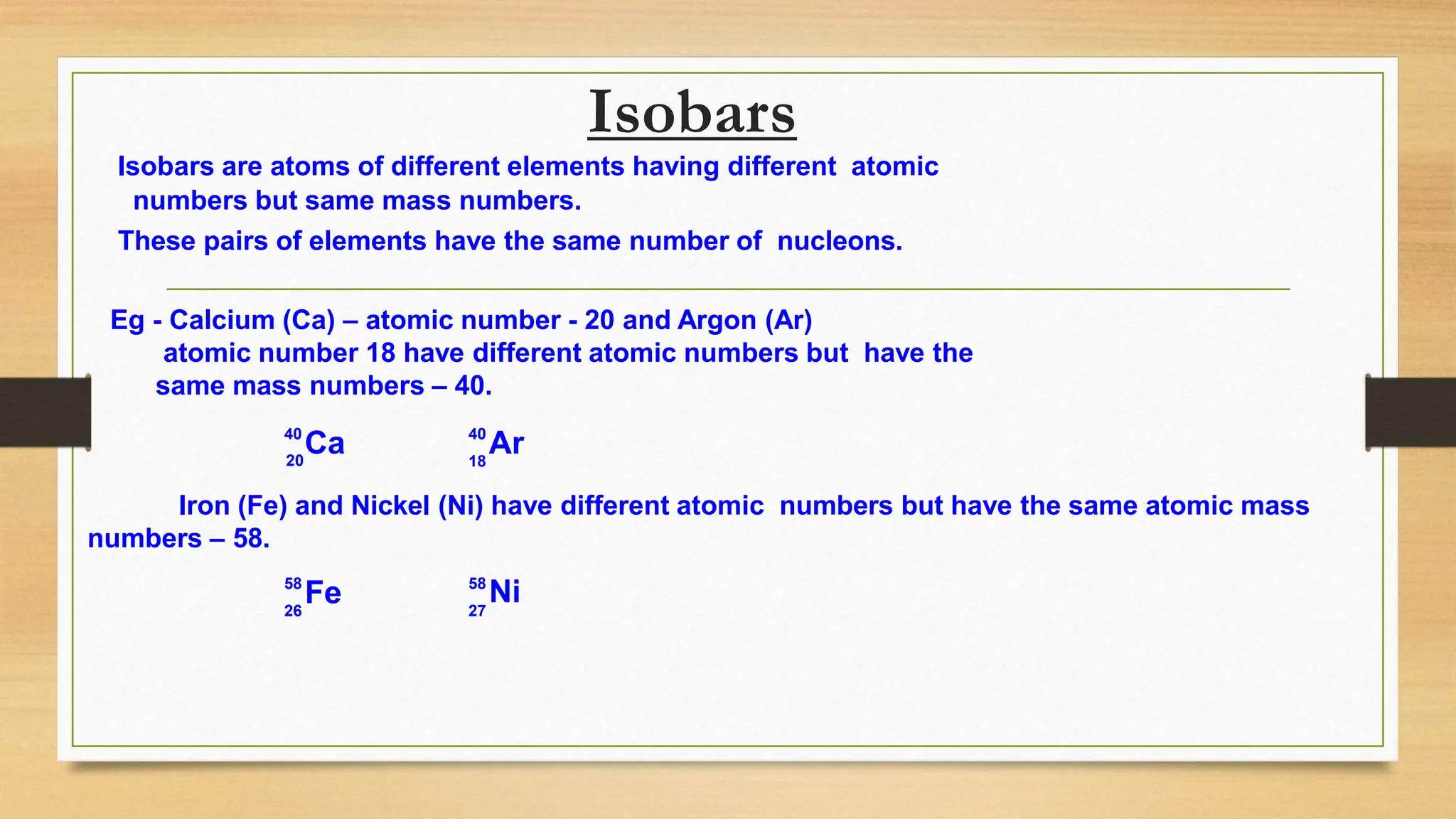
This document discusses the structure of the atom, highlighting the three types of subatomic particles: electrons, protons, and neutrons, along with their charges and mass. It covers the historical discoveries of these particles by scientists like Thomson, Rutherford, and Chadwick, and details various atomic models, emphasizing Rutherford's and Bohr's models, which describe the arrangement of electrons and the nucleus. The document also touches on concepts like atomic number, mass number, isotopes, and valency, providing a comprehensive overview of atomic structure and behavior.