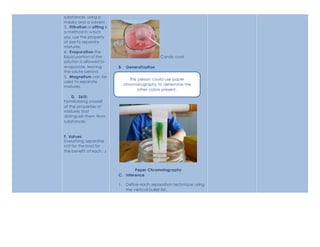
The document outlines a semi-detailed lesson plan for teaching students about separation techniques of mixtures. It includes learning objectives, content, concepts, procedures, and assessments. Students will learn about different separation methods like distillation, chromatography, filtration, evaporation and magnetism through pictures and examples. They will then work in groups to demonstrate one technique creatively and be evaluated on accuracy and presentation skills. The goal is for students to understand how to identify and separate mixtures into their individual substances.