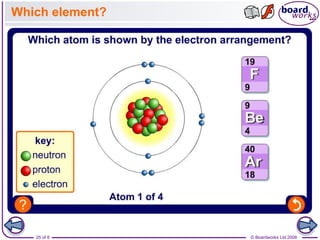
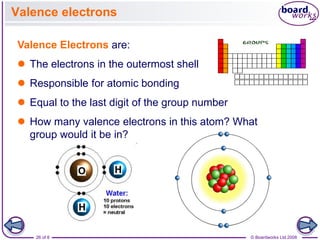
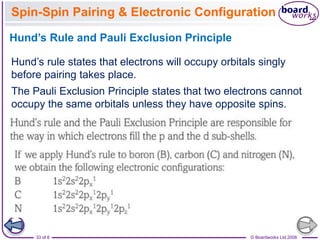
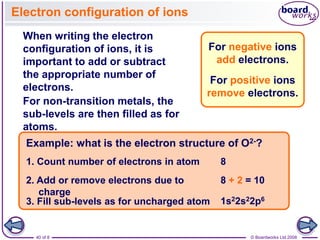
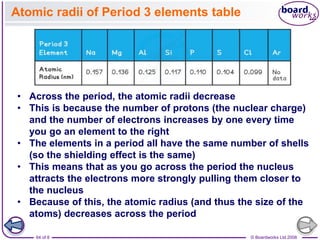
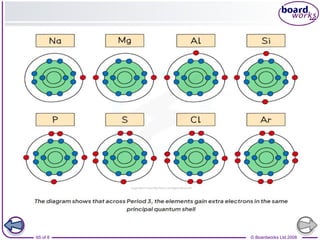
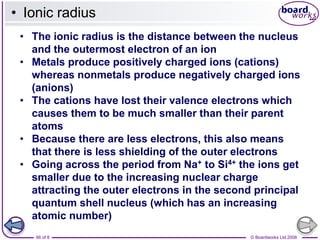
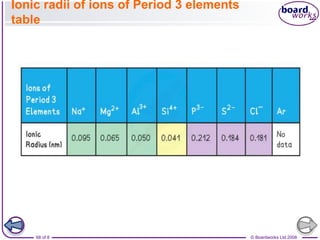
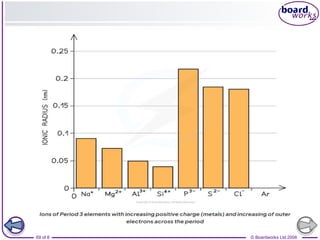
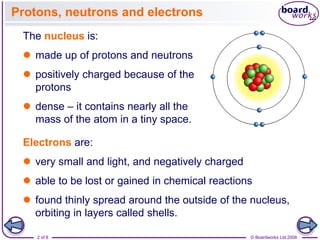
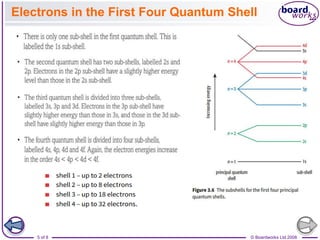
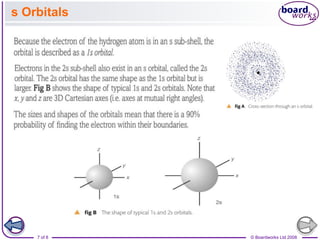
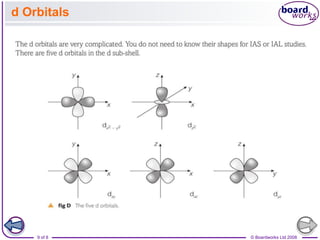
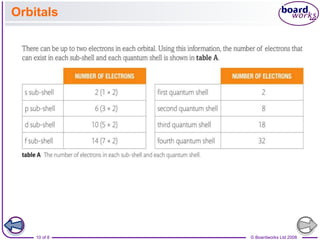
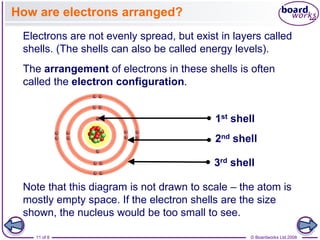
The document discusses atomic structure, detailing the roles and arrangement of protons, neutrons, and electrons within an atom, including concepts like electron shells and configurations. It explains how to create Bohr models of atoms, the filling order of subshells according to the Aufbau principle, and how ions are formed and characterized. Additionally, it examines ionization energy trends and factors affecting these energies, providing insights into electronic configurations and the stability of various elements.







![© Boardworks Ltd 2008
21 of 8
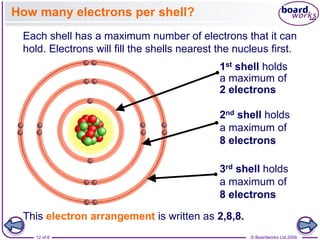
Electronic configurations
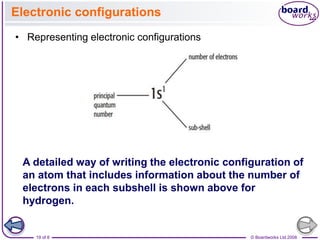
• The electronic
configurations of some of
the elements after argon
are shown in Table 3.6.
• In this table part of the
electronic configuration of
each element is
represented by [Ar].
• This ‘noble gas core’
represents the electronic
configuration of argon:
1s2 2s2 2p6 3s2 3p6.
• This method is a
shorthand way of writing
electronic structures of
atoms with many
electrons.](https://image.slidesharecdn.com/4electronandelectronconfiguration-230219022101-b15cf001/85/4Electron-and-Electron-Configuration-pptx-21-320.jpg)
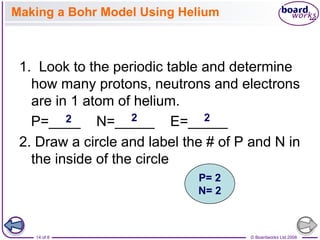
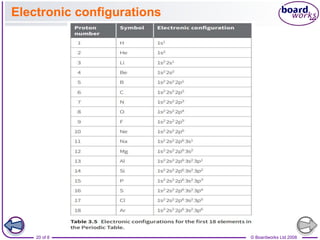
![© Boardworks Ltd 2008
22 of 8
Electronic configurations
■ Electronic configuration of potassium
Potassium has the electronic structure 1s2 2s2 2p6 3s2 3p6
4s1. The outer electron goes into the 4s subshell rather than
the 3d subshell because the 4s is below the 3d in terms of its
energy
■ Filling the 3d subshell
After calcium, a new subshell becomes occupied.
The next electron goes into a 3d subshell rather than a 4p
subshell.
So scandium has the electronic configuration [Ar] 3d1 4s2.
This is because electrons occupy the orbitals with the lowest
energy – the 3d subshell is just above the 4s subshell but
below the 4p subshell.
This begins a pattern of filling the 3d subshell ending with
zinc.
Zinc has the electronic configuration [Ar] 3d10 4s2.](https://image.slidesharecdn.com/4electronandelectronconfiguration-230219022101-b15cf001/85/4Electron-and-Electron-Configuration-pptx-22-320.jpg)
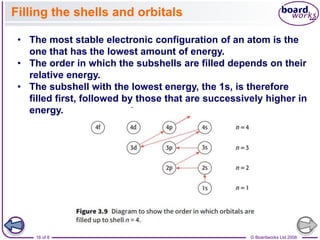
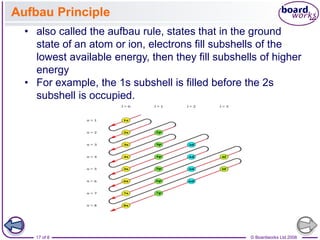
![© Boardworks Ltd 2008
23 of 8
Electronic configurations
■ Chromium and copper
The electronic configurations of chromium and copper do
not follow the expected pattern.
Chromium has the electronic configuration [Ar]3d5 4s1
(rather than the expected [Ar]3d4 4s2).
Copper has the electronic configuration [Ar]3d10 4s1 (rather
than the expected [Ar]3d9 4s2).
■ Gallium to krypton
The electrons add to the 4p subshell because this is the next
highest energy level above the 3d.](https://image.slidesharecdn.com/4electronandelectronconfiguration-230219022101-b15cf001/85/4Electron-and-Electron-Configuration-pptx-23-320.jpg)