This document provides instructions and information for a science class. It includes:
1. A list of materials needed for the lesson including a pencil, periodic table, and power notes.
2. Reminders about due dates for assignments including a binder check, minimum days, and the end of the quarter.
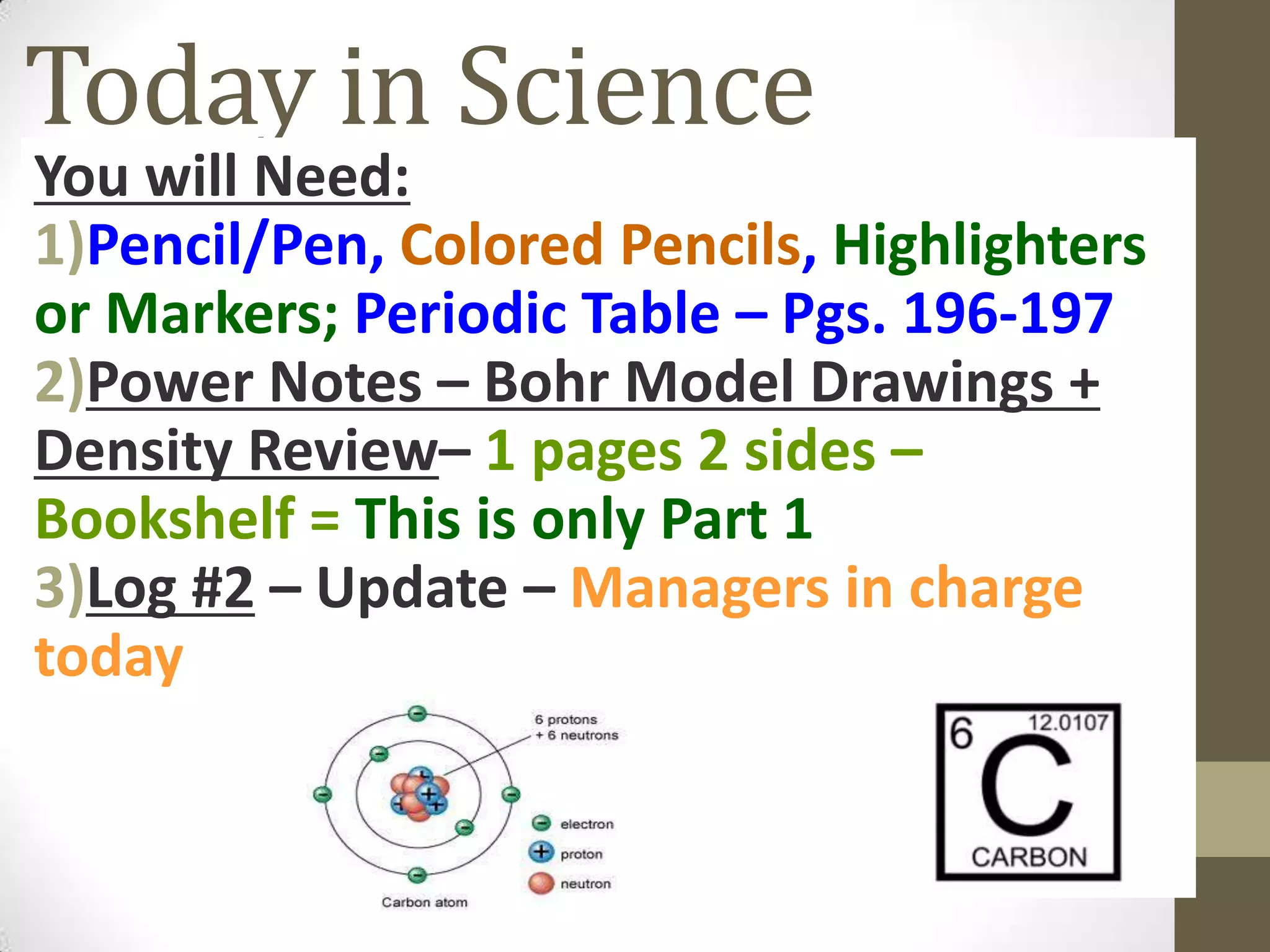
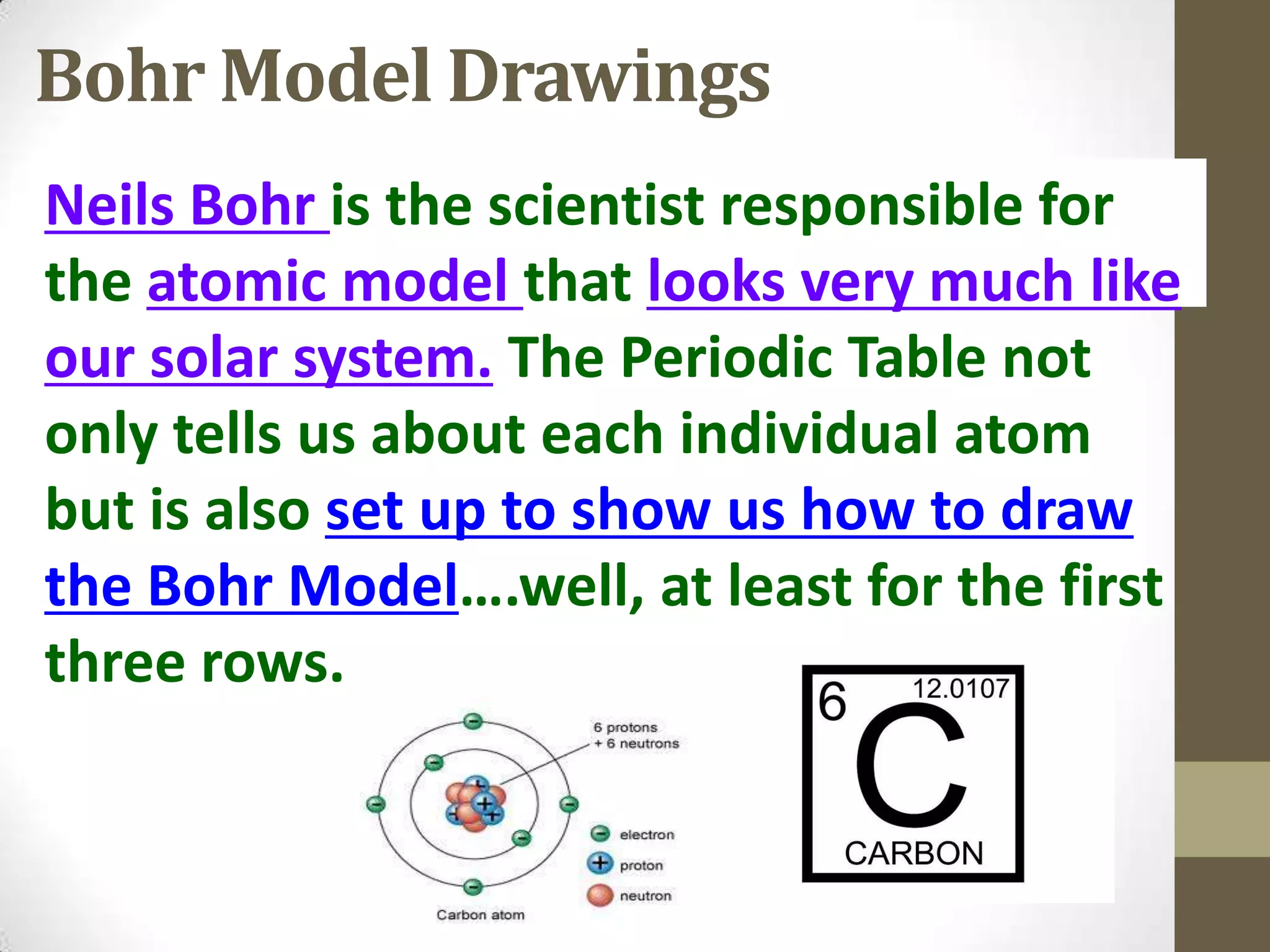
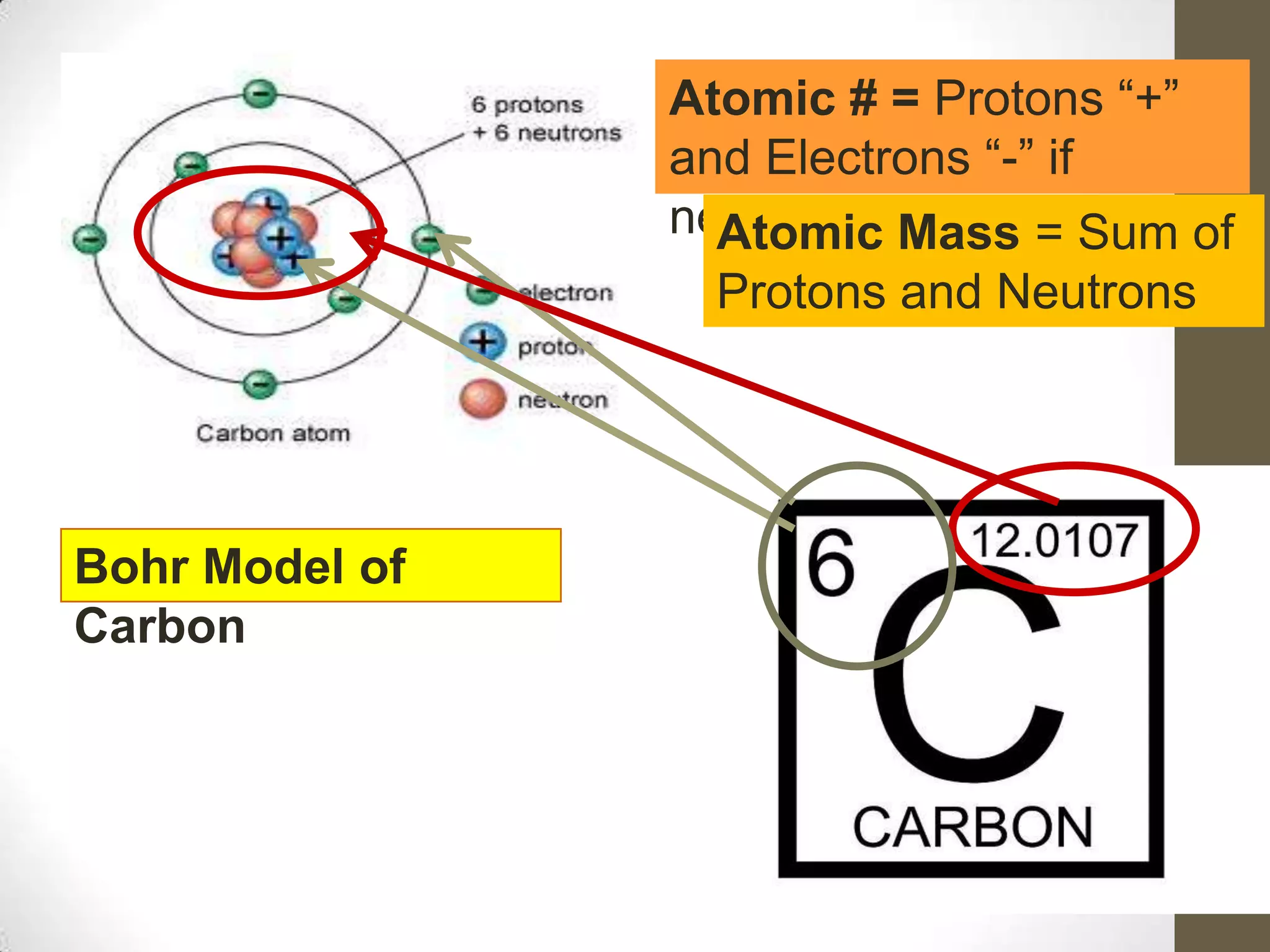
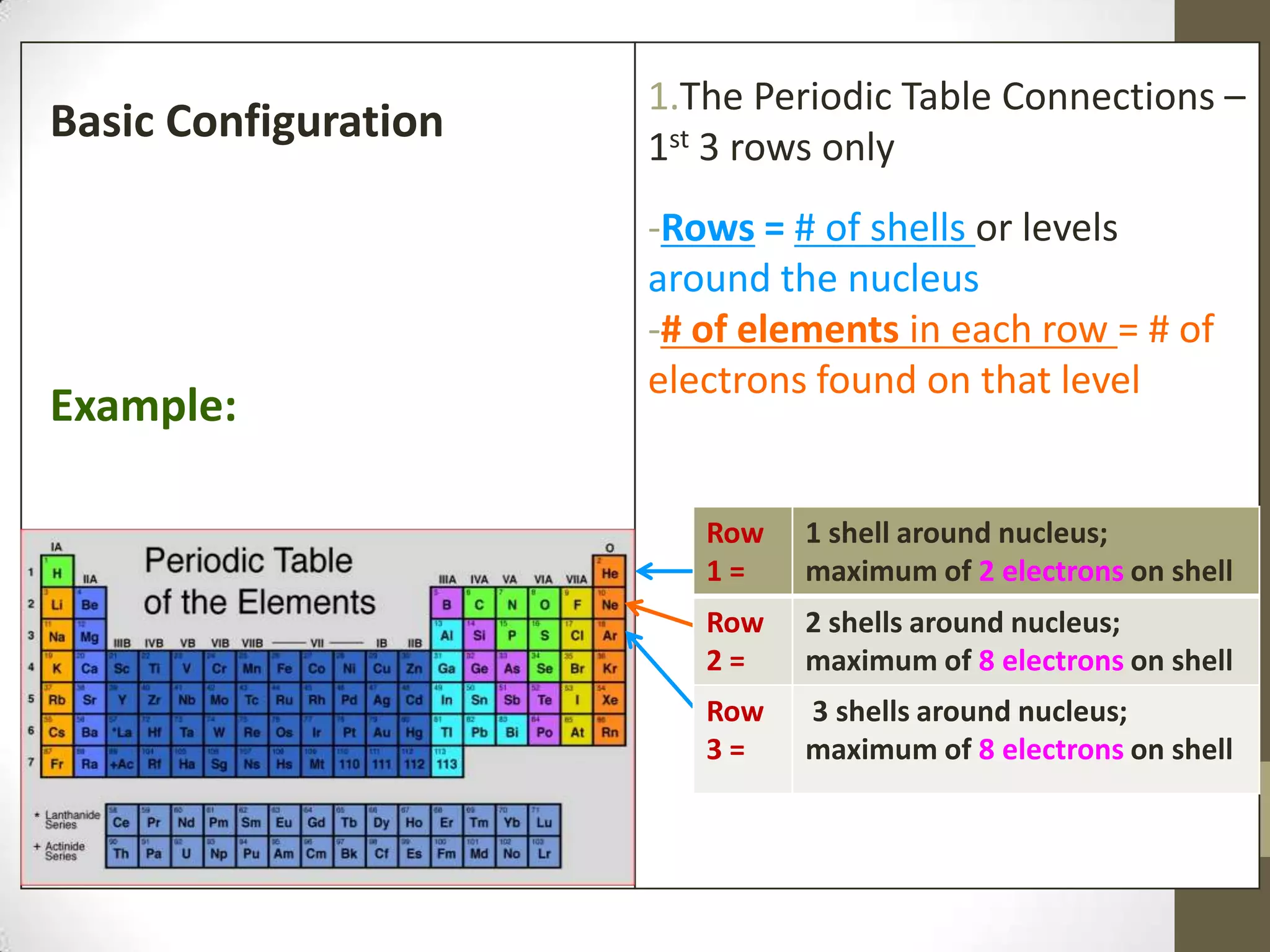
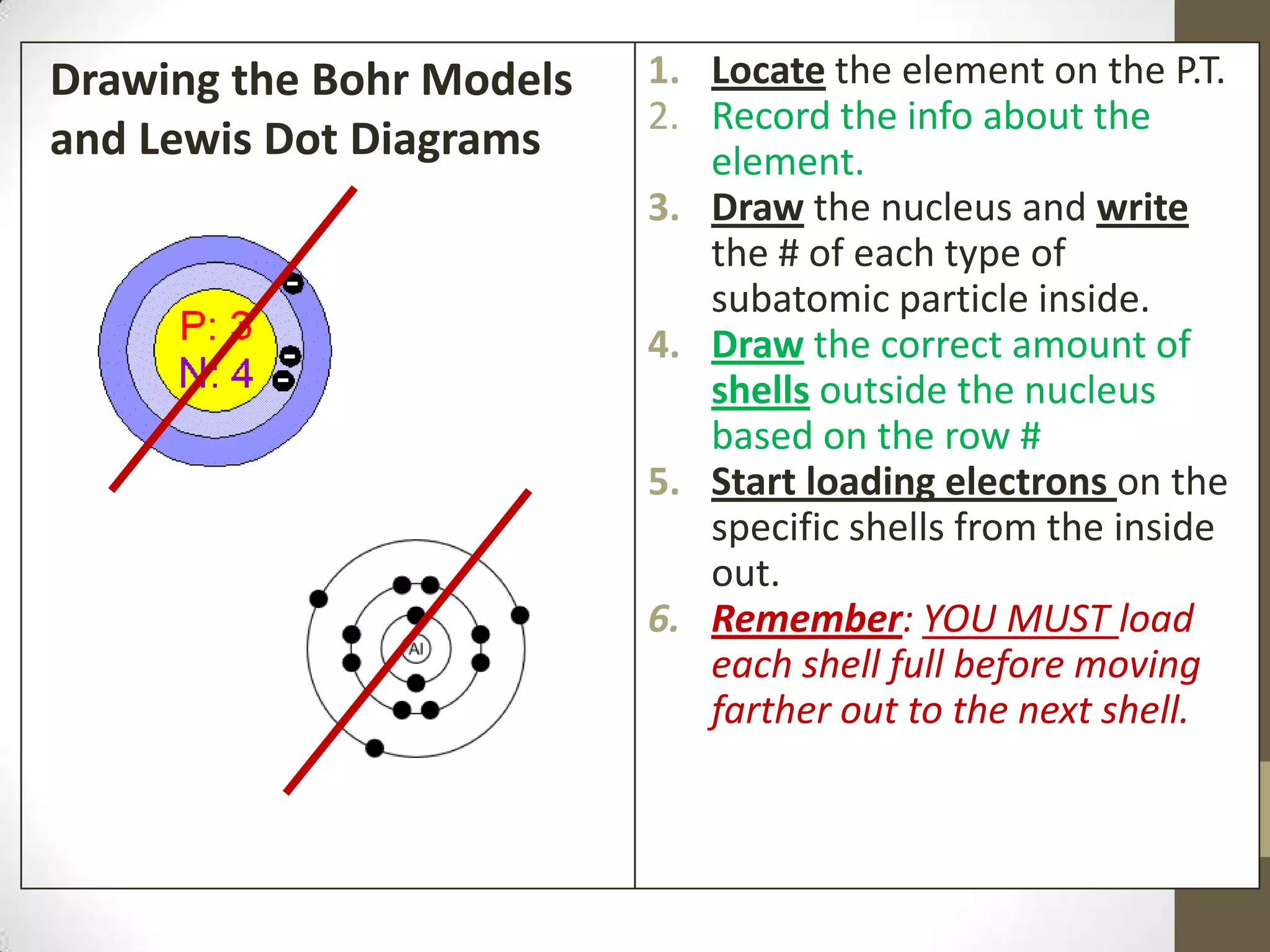
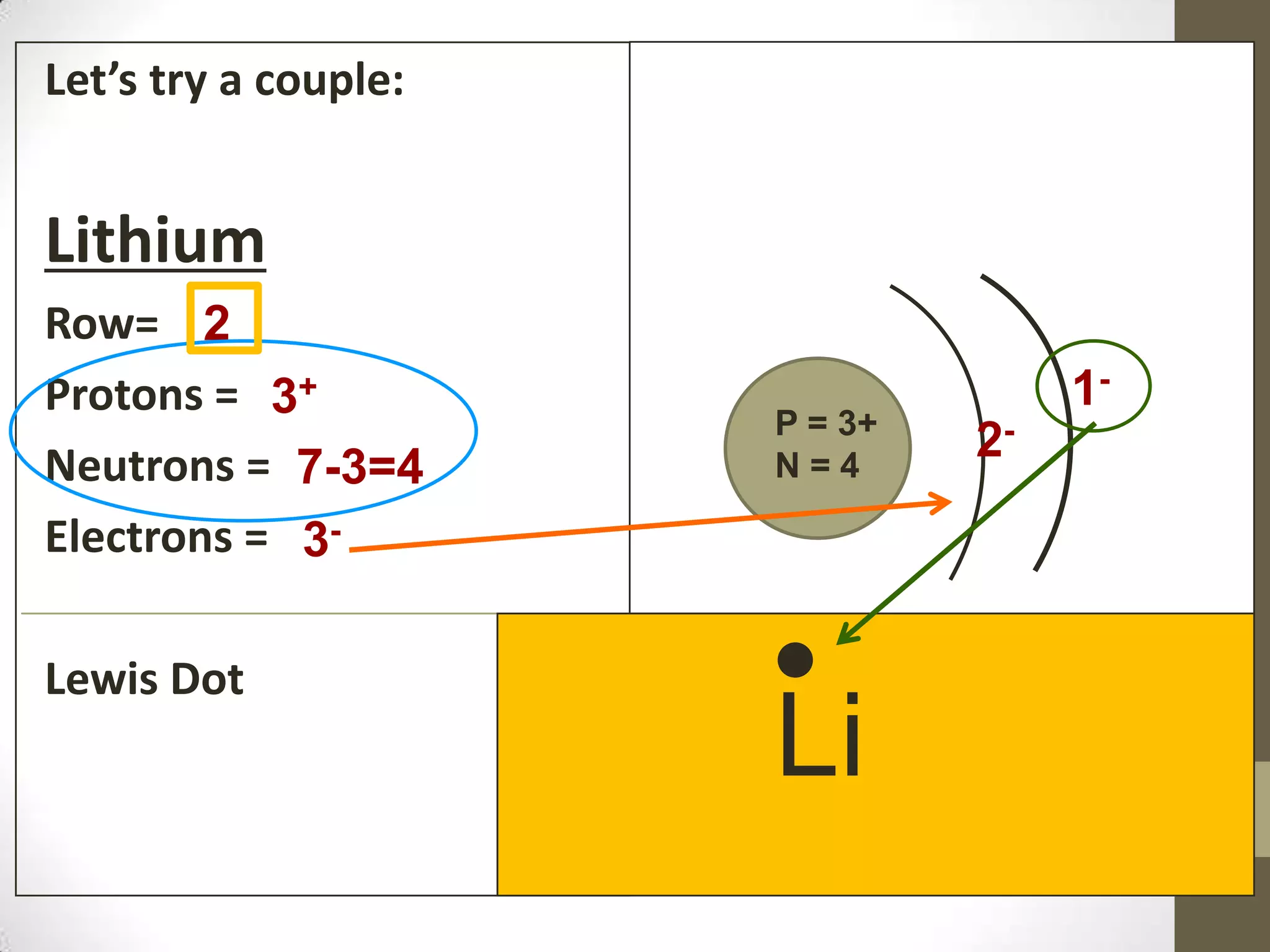
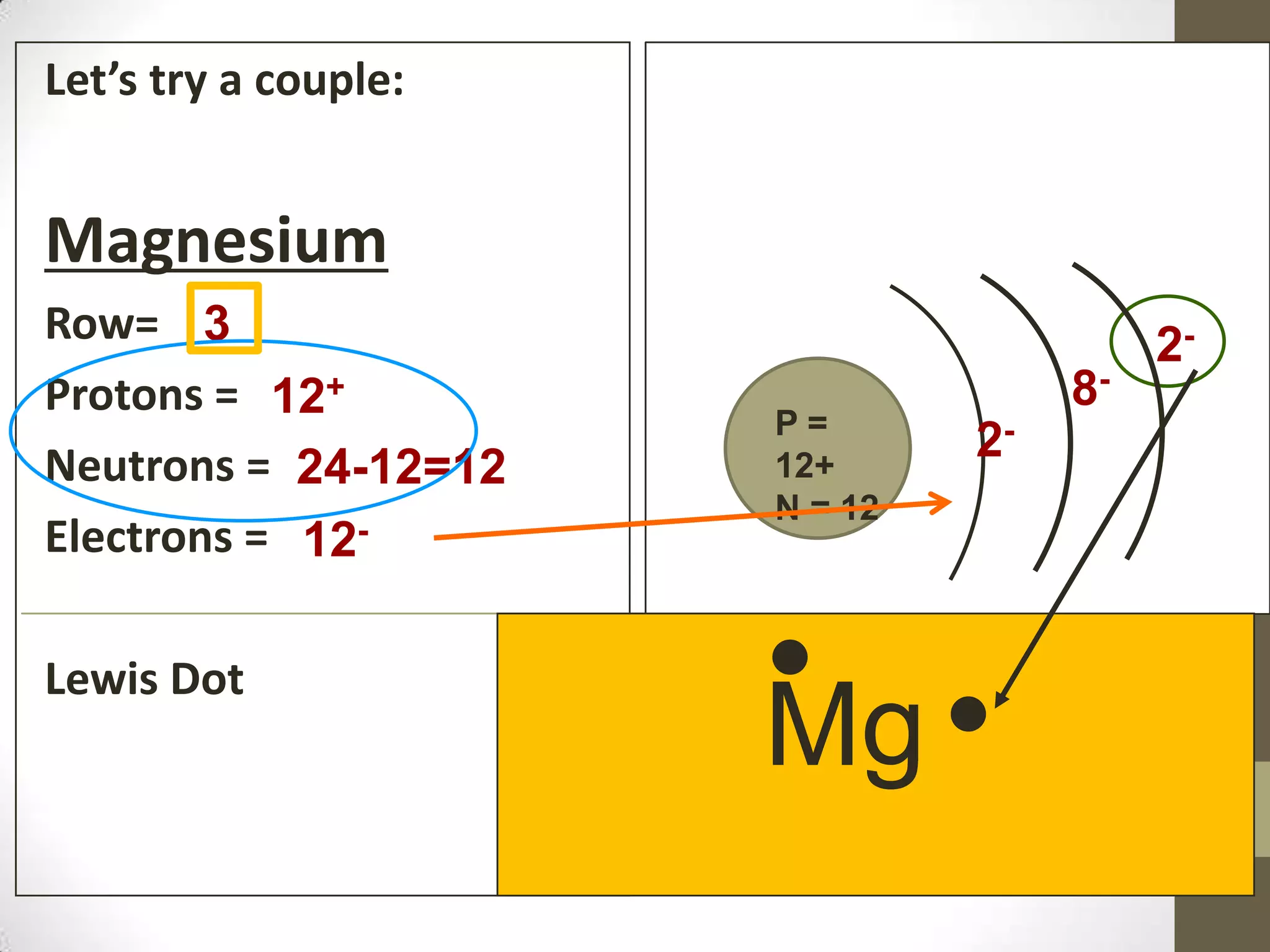
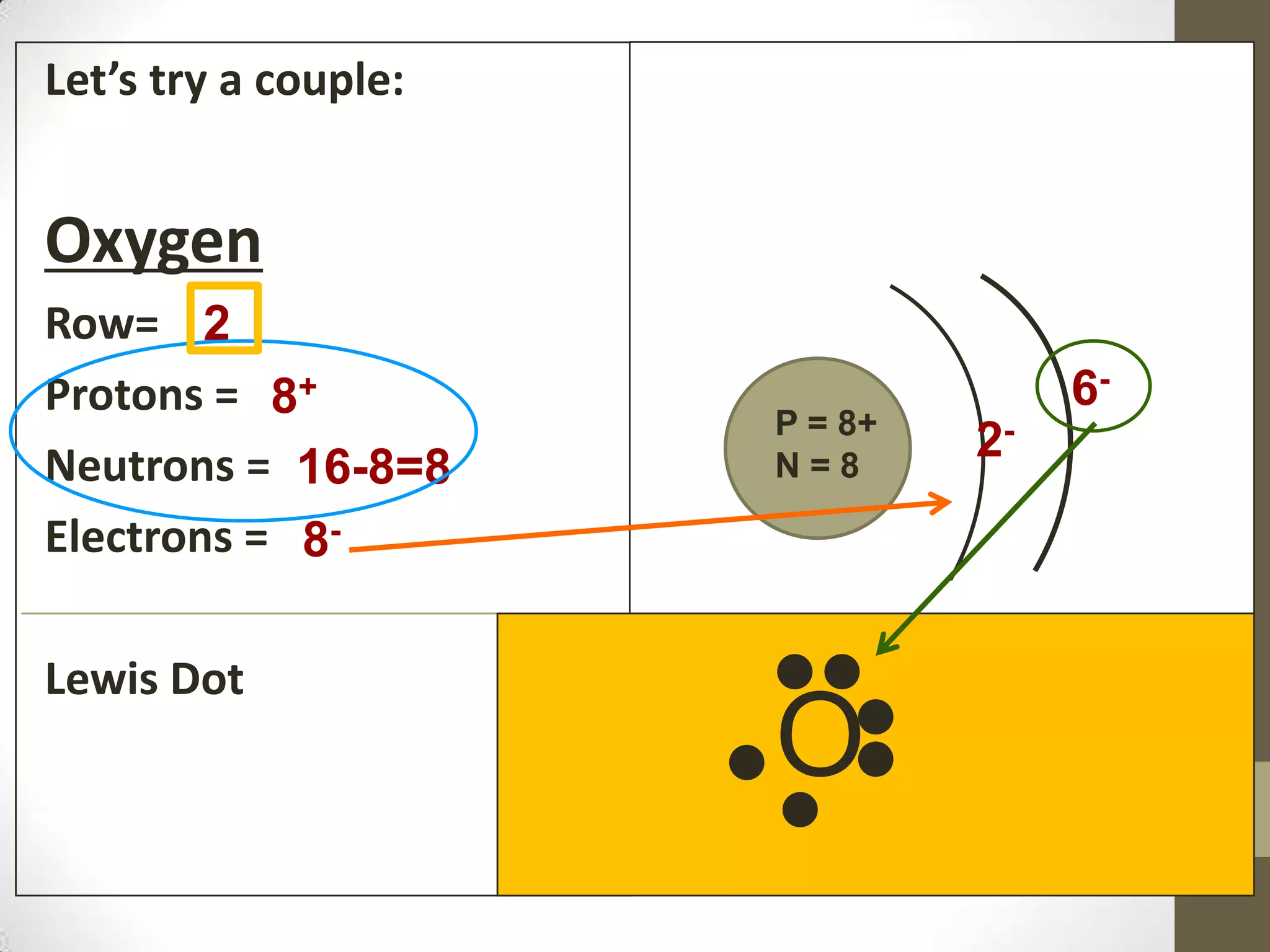
3. Information on drawing Bohr models including an explanation of Neils Bohr's atomic model and how it relates to the periodic table and electron configuration. Examples are provided for drawing Bohr models of carbon, lithium, and magnesium.
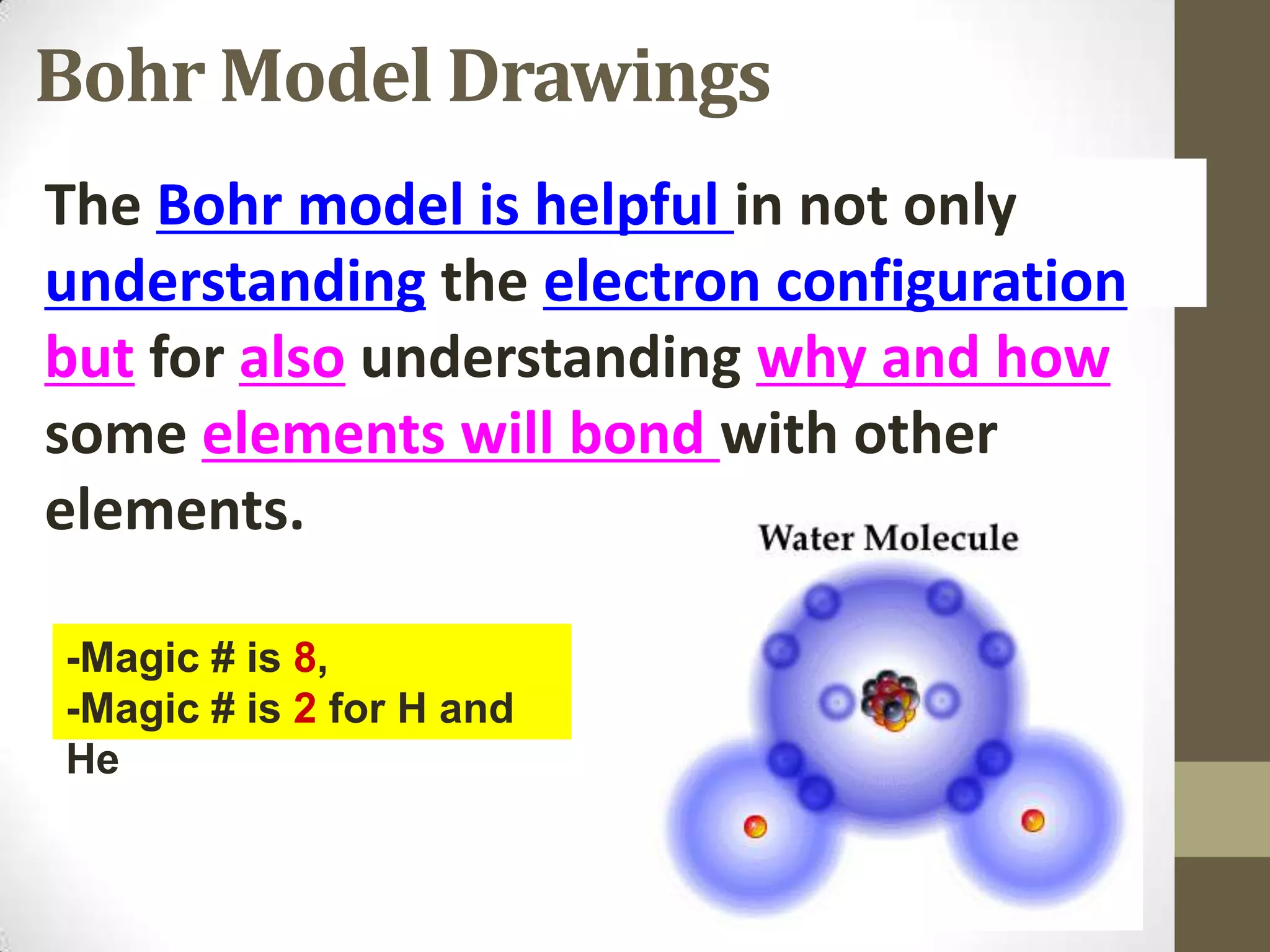
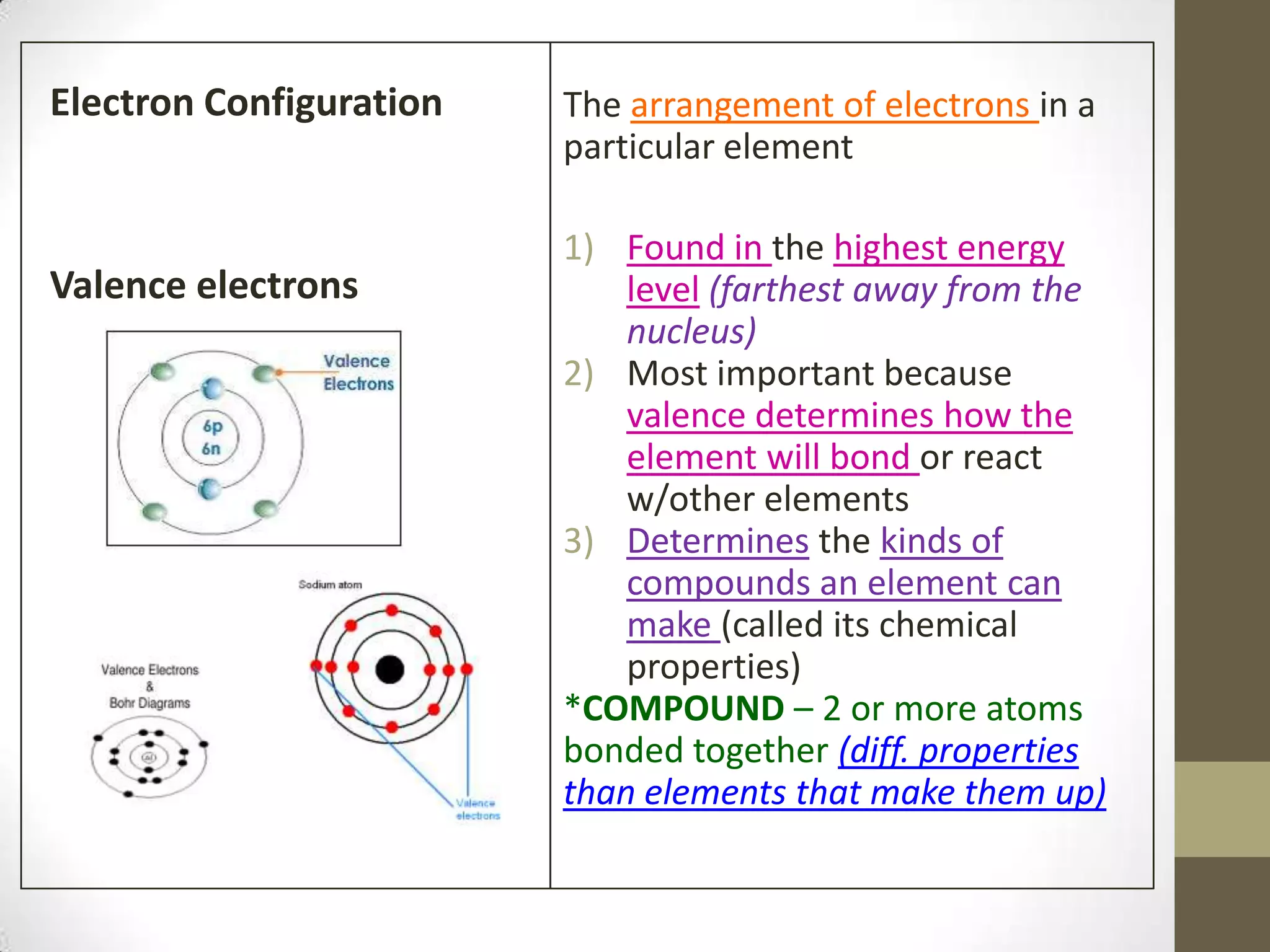
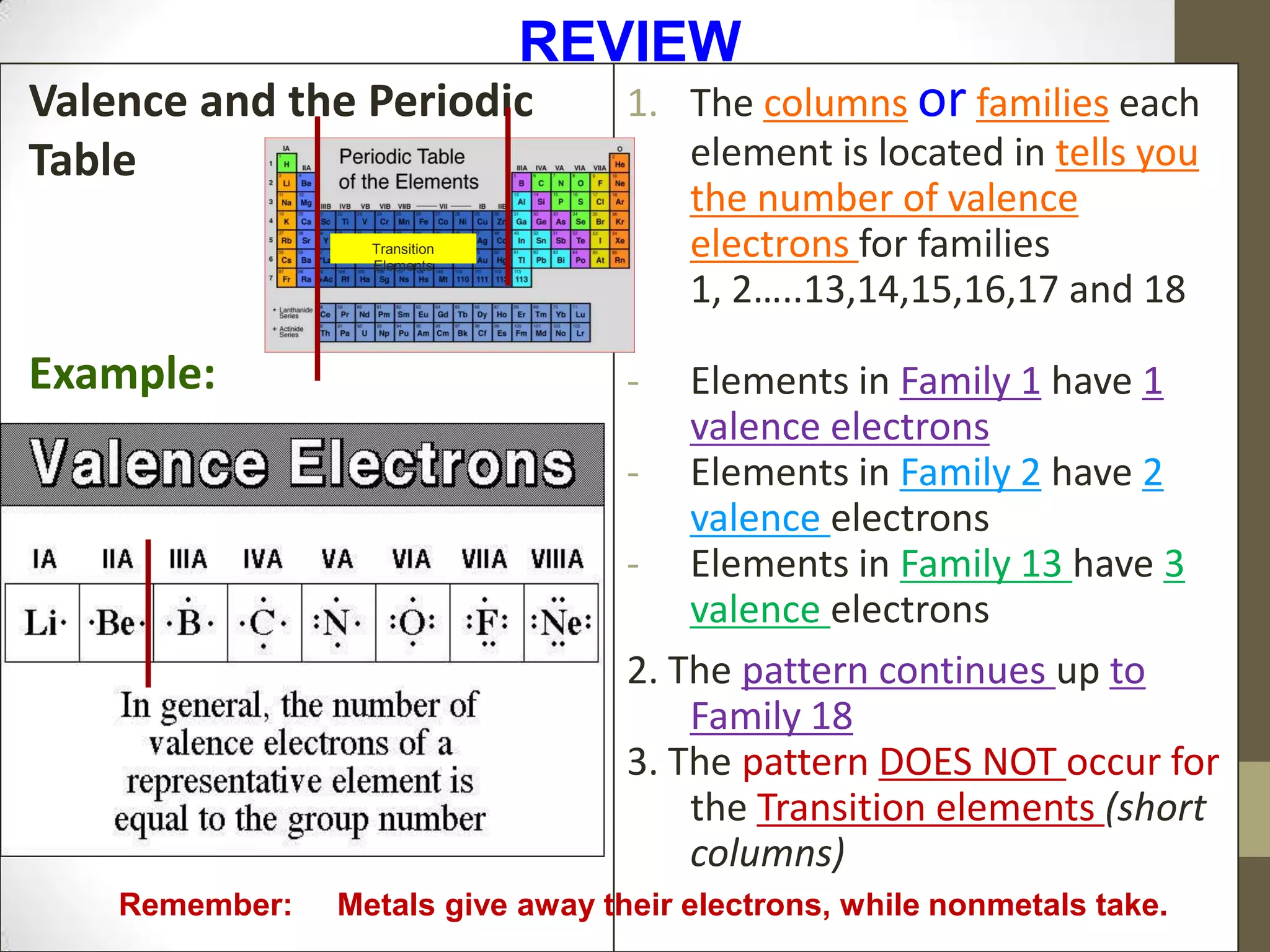
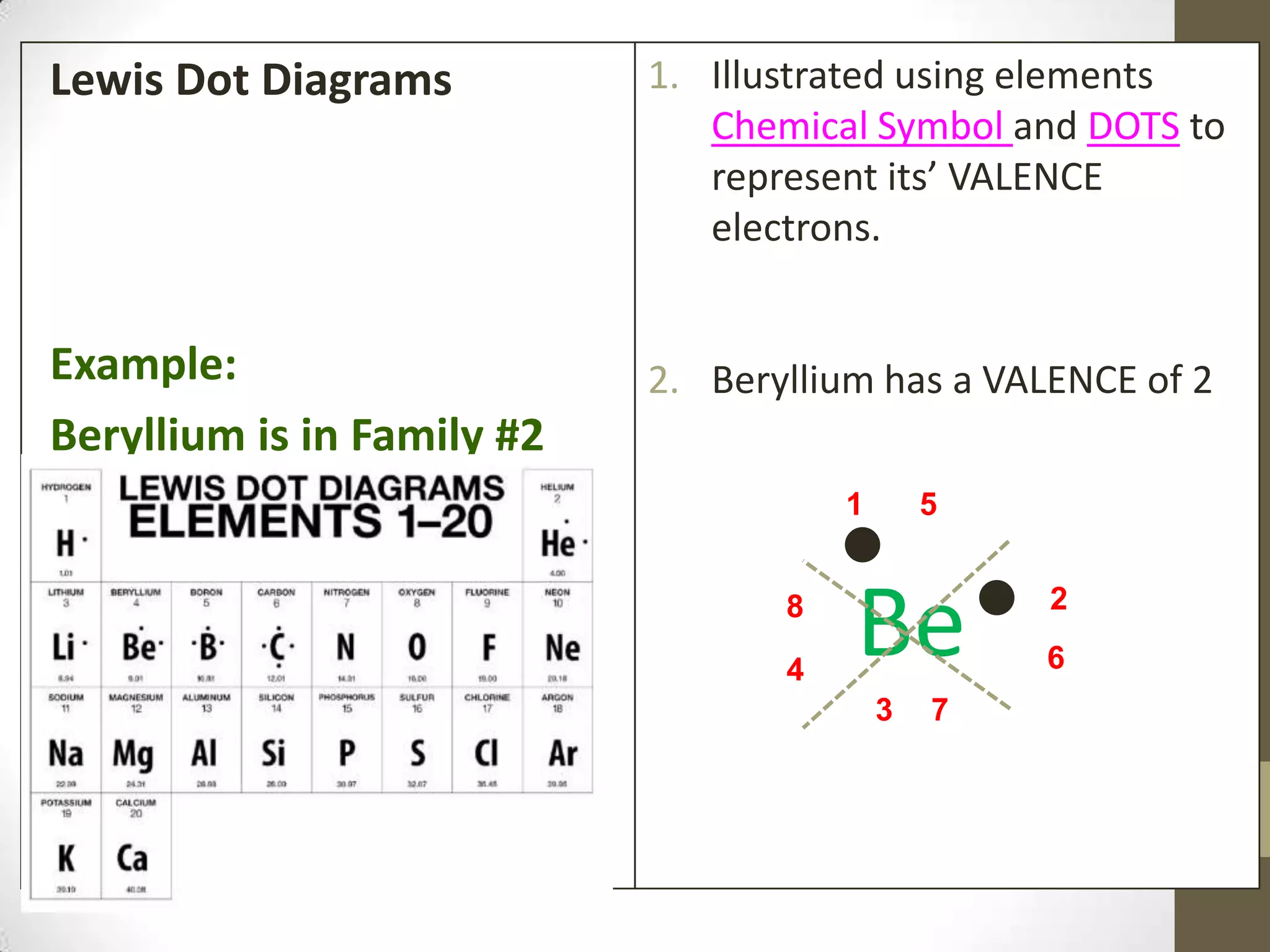
4. Details on electron configuration, valence electrons, and using the periodic table to determine the number of valence electrons. Lewis dot diagrams are also explained.