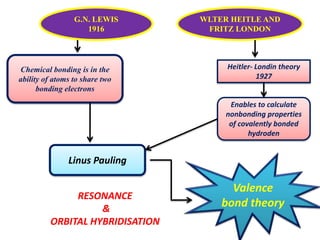
The document discusses valence bond theory and crystal field theory as they apply to coordination compounds.
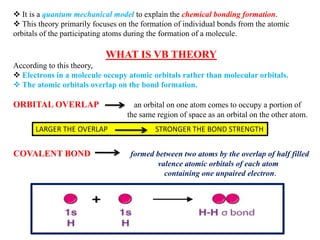
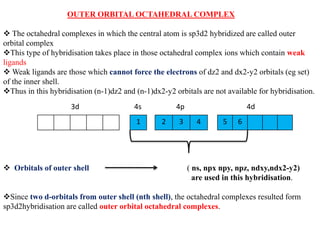
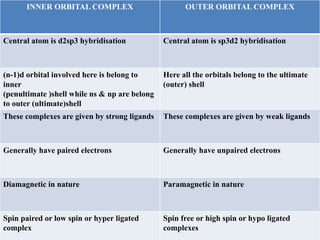
Valence bond theory describes chemical bonding as occurring through the overlap of atomic orbitals. It can explain octahedral complexes through d2sp3 or sp3d2 hybridization of the metal's orbitals. Crystal field theory postulates that ligands exert an electrostatic field that splits the metal's d-orbitals into two energy levels. In an octahedral field, the eg orbitals have higher energy than the t2g orbitals. Complexes with weak ligands that cause little splitting tend to be high spin, while those with strong ligands that cause large splitting are low spin.







![electron configuration of the transition- metal ion.
Co3+: [Ar] 3d6
We then look at the valence-shell orbitals and note that the 4s and 4p orbitals are empty.
Co3+: [Ar] 3d6 4s0 4p0
Concentrating the 3d electrons in the dxy, dxz, and dyz orbitals in this subshell gives the
following electron configuration.
3dx
2
-y
2, 3dz
2, 4s, 4px, 4py and 4pz orbitals are then mixed to form a set of empty d2sp3 orbitals
that point toward the corners of an octahedron
Each of these orbitals can accept a pair of nonbonding electrons from a neutral
NH3 molecule to form a complex in which the cobalt atom has a filled shell of valence
electrons.](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-10-320.jpg)






![ Co3+ does not contain any empty 3d orbital . Now according to VBT, in the
presence of CN- (strong ligand) the electrons in 3d orbitals are force to pair up
against Hund’s rule in order to make room for electrons donated by the ligands.
Now there are six hybrid d2sp3 orbitals which can accept six electron pair form
ligands.
d2sp3 hybridization gives octahedral geometry
It has no unpaired electrons, so it is diamagnetic in nature.
The inner (n-1)d orbitals are taking part in bond formation, hence it is inner
complex ion
Eg, [Co(CN)6]3- ,[ Co(No2)] 3-](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-17-320.jpg)



![Crystal field theory (CFT) describes the breaking of orbital degeneracy
in transition metal complexes due to the presence of ligands.
CFT qualitatively describes the strength of the metal-ligand bonds.
POSTULATES OF CFT
The ligands are point charges which are either ions [ F-, Cl-, CN-, etc] or neutral
molecules [NH3, H2O,etc] with negative poles oriented towards metal cation
The transition metal cation is surrounded by the ligand with lone pair of
electrons
The attraction between metal cation and ligands is purely ionic
The valence electrons of metal will be repelled by negative field of ligands so these
electrons occupy those d-orbitals which have their lobe away from direction of the
ligand
The colour of transition metal complexes can be explained in terms of electronic
transition between the various d-orbitals of different energy
The magnetic properties can be explained in terms of splitting of d-orbitals in
different crystal field.
Different crystal fields will have different effect of the relative energies of the five
d-orbitals.](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-23-320.jpg)
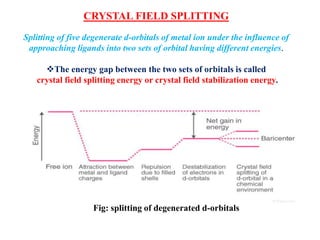




![HIGH SPIN AND LOW SPIN COMPLEXES
The complexion with the greater number of unpaired electrons is known as the high spin
complex
The low spin complex contains the lesser number of unpaired electrons.
High spin complexes are expected with weak field ligands whereas the crystal field
splitting energy is small Δ.
The opposite applies to the low spin complexes in which strong field ligands cause
maximum pairing of electrons in the set of three t2 atomic orbitals due to large Δo.
High spin Maximum number of unpaired electrons.
Low spin Minimum number of unpaired electrons
[Co(CN)6]3- – Low spin complex
[CoF6]3- – High spin complex](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-29-320.jpg)
![CRYSTAL FIELD STABILIZATION ENERGY
The energy of the electron configuration in the ligand field minus the energy of the
electronic configuration in the isotropic field.
CFSE = ∆E = Eligand field - Eisotopic field .
The energy difference between the eg and t2g levels is given as ∆ or 10Dq
It states that each electron that goes into the lower t2g level stabilizes the system by an
amount of -4Dq and the electron that goes into eg level destabilizes the system by +6Dq.
That is the t2g is lowered by 4Dq and the eg level is raised by +6Dq.
Paring in
Degenerate
d - orbital
[ (t2g electrons) × (- 4Dq) ] + [ (eg electrons) × ( 6Dq) + paring energy
For example, the net change in energy for d5 and d10 systems will be zero as
shown below.
d5 :- 3(-4Dq) + 2(+6Dq) = -12Dq + 12Dq = 0
d10 :- 6(-4Dq) + 4(+6Dq) = -24Dq + 24Dq = 0](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-30-320.jpg)







![CHARGE ON THE METAL ION:
Increasing the charge on a metal ion has two effects:
the radius of the metal ion decreases,
and negatively charged ligands are more strongly attracted to it.
Both factors decrease the metal–ligand distance, which in turn causes the
negatively charged ligands to interact more strongly with the d orbitals.
Consequently, the magnitude of Δo increases as the charge on the metal ion
increases.
Typically, Δo for a tripositive ion is about 50% greater than for the dipositive ion
of the same metal;
for example,
for [V(H2O)6]2+, Δo = 11,800 cm−1;
for [V(H2O)6]3+, Δo = 17,850 cm−1.
PRINCIPAL QUANTUM NUMBER OF THE METAL:
For a series of complexes of metals from the same group in the periodic table
with the same charge and the same ligands, the magnitude of Δo increases with
increasing principal quantum number: Δo (3d) < Δo (4d) < Δo (5d).](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-38-320.jpg)
![The data for hexamine complexes of the trivalent Group 9 metals illustrate this
point:
[Co(NH3)6]3+: Δo = 22,900 cm−1
[Rh(NH3)6]3+: Δo = 34,100 cm−1
[Ir(NH3)6]3+: Δo = 40,000 cm−1
The increase in Δo with increasing principal quantum number is due to the larger
radius of valence orbitals down a column.
In addition, repulsive ligand–ligand interactions are most important for
smaller metal ions.
This results in shorter M–L distances and stronger d orbital–ligand interactions
NATURE OF THE LIGAND:
Ligands which cause only a small splitting are called weak ligands and those
which bring a large splitting is called strong ligands.
It is possible to arrange the ligands according to magnitude of ∆ observed
with metal ion.](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-39-320.jpg)



![For example,
The complex [Cr(NH3)6]3+ has strong-field ligands and a relatively large Δo.
Consequently, it absorbs relatively high-energy photons, corresponding to
blue-violet light, which gives it a yellow color.
A related complex with weak-field ligands, the [Cr(H2O)6]3+ ion, absorbs
lower-energy photons corresponding to the yellow-green portion of the visible
spectrum, giving it a deep violet color](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-43-320.jpg)
![It is clear that the environment of the transition-metal ion, which is determined by
the host lattice, dramatically affects the spectroscopic properties of a metal ion.
Fig: Gem-quality crystals of ruby and emerald. The colours of both minerals are due to
the presence of small amounts of Cr3+ impurities in octahedral sites in an otherwise
colourless metal oxide lattice.
RUBY :
Cr–O distances are relatively short because of the constraints of the host lattice, which
increases the d orbital–ligand interactions and makes Δo relatively large.
Consequently, rubies absorb green light and the transmitted or reflected light is red,
which gives the gem its characteristic color
EMERALD :
Cr–O distances are longer due to relatively large [Si6O18]12− silicate rings; this results in
decreased d orbital–ligand interactions and a smaller Δo.
Consequently, emeralds absorb light of a longer wavelength (red), which gives the gem
its characteristic green color](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-44-320.jpg)

![The size of the magnetic moment of a system containing unpaired electrons is related
directly to the number of such electrons
The greater the number of unpaired electrons, the larger the magnetic moment.
Magnetic susceptibility measures the force experienced by a substance in a magnetic
field.
When we compare the weight of a sample to the weight measured in a magnetic
field , paramagnetic samples that are attracted to the magnet will appear heavier
because of the force exerted by the magnetic field.
We can calculate the number of unpaired electrons based on the increase in weigh
From this experiment, the measured magnetic moment of low-
spin d6 [Fe(CN)6]4− ion confirms that iron is diamagnetic, whereas the high-
spin d6 [Fe(H2O)6]2+ complex has four unpaired electrons with a magnetic moment
that confirms this arrangement.
Gouy’s Balance
method](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-46-320.jpg)

![As an example,
Fe prefers to exist as Fe3+ and is known to have a coordination number of six.
Since the configuration of Fe3+ has five d electrons, we would expect to see five
unpaired spins in complexes with Fe.
This is true for [FeF6]3-; however, [Fe(CN)6]3−only has one unpaired electron,
making it a weaker magnet.
we expect CN−CN− ligands to have a stronger electric field than that
of F−F− ligands, so the energy differences in the d-orbitals should be greater for the
cyanide complex.](https://image.slidesharecdn.com/coodinationchemistryaesir-210131033838/85/Coodination-chemistry-48-320.jpg)













