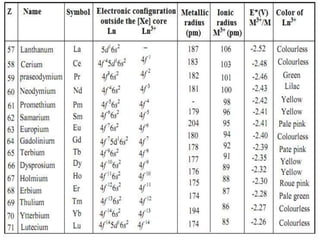
The document discusses the oxidation states of lanthanides. It states that:
1) All lanthanides most commonly exhibit a +3 oxidation state, but some can also be +2, +4, or lower states depending on electronic configuration.
2) The most stable oxidation state is generally +3 due to the strong attraction of the 4f electrons to the nucleus.
3) Elements in other oxidation states act as strong reducing or oxidizing agents as they try to attain the +3 state.


![LANTHANIDES
Rare Earth Element
Occurrence: 3×10-4 .
% of earth crust
Available in monzite
sand as lanthanide
orthophophates
Norwegain
mineralogist victor
Gold schmidt in 1925
Z= 58 to 71
Fifteen metallic elements
( La to Lu)
Highly dense elements
(6.1 to 9.8g per cc)
Mp: 800 – 1600
Bp: 1200 - 3500
Valance electrons lies in
4f orbitals
Electronic configuration
[Xe]4f1-145d0-16s2](https://image.slidesharecdn.com/lanthanideoxidation-210131040247/85/Lanthanide-oxidation-3-320.jpg)








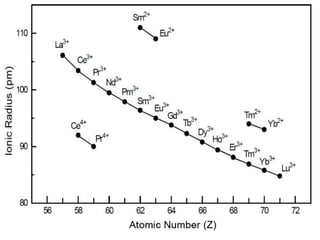
![OCCURRENCE OF +2 OXIDATION STATE
Eu2+ [4f7 ], Sm2+ [4f6 ], Yb2+ [4f14] clear influences of
electronic shell structure
Europium (Z=63) [Xe] 4f7 6s2 half-filled
4f7 configuration and hence it readily forms Eu2+ion.
Eu2+ then changes to the common oxidation states of lanthanides (+3) and
forms Eu3+, acting as a strong reducing agent.
Ytterbium (Z=70) fully filled f-orbital
It has similar reasons for being a strong reducing agent, in the Yb2+ state.](https://image.slidesharecdn.com/lanthanideoxidation-210131040247/85/Lanthanide-oxidation-12-320.jpg)