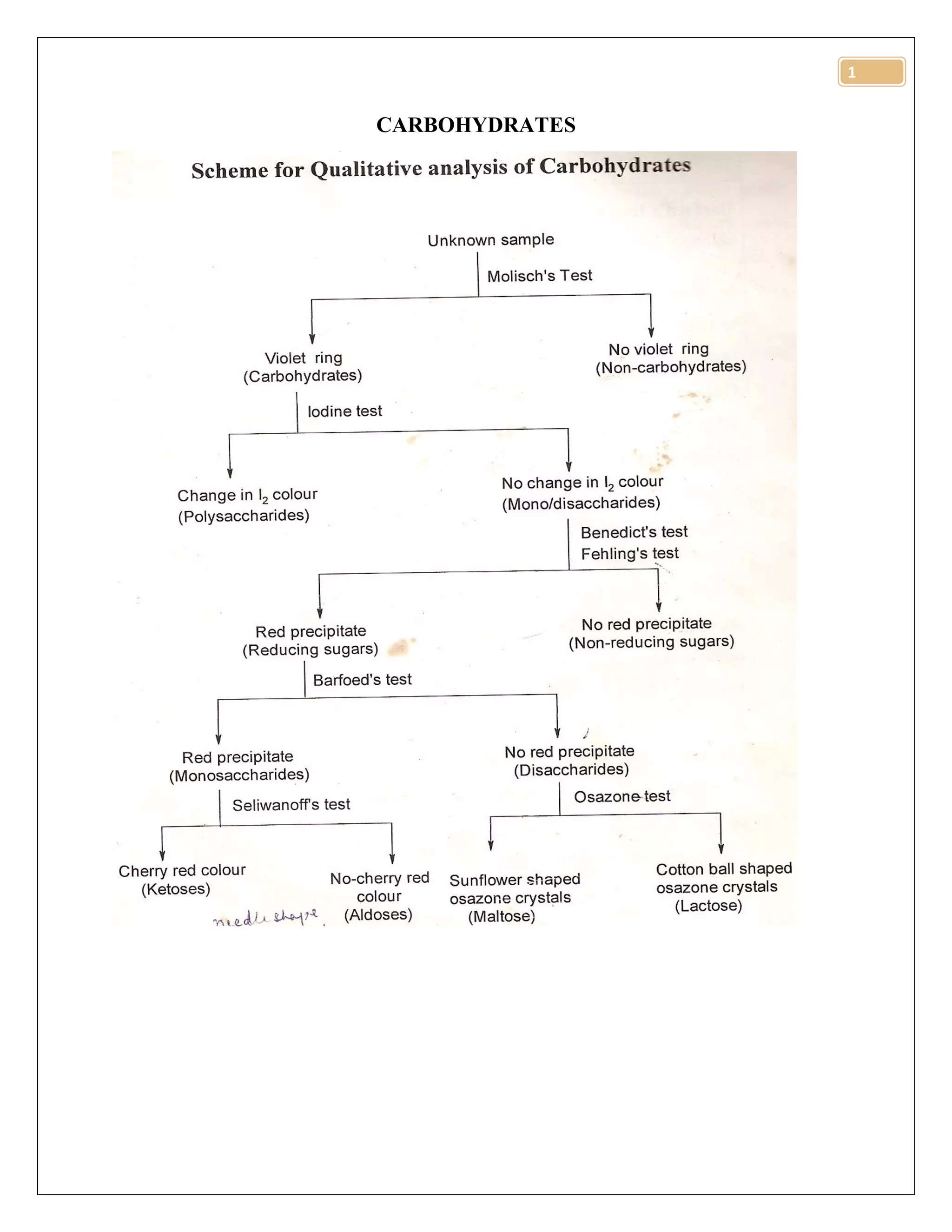
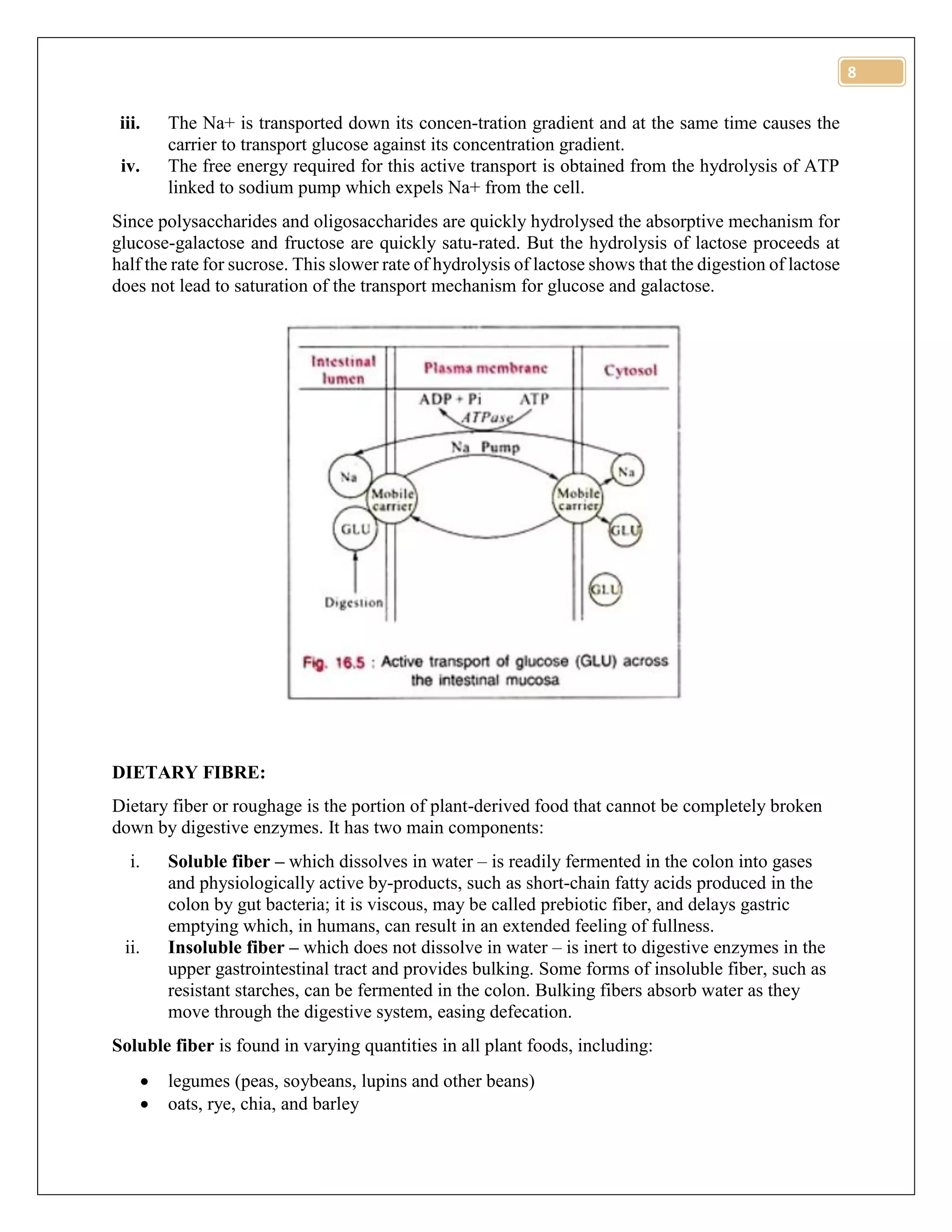
The document discusses various methods for quantitative analysis of carbohydrates, including Lane-Eynon, Munson-Walker, and Nelson-Somogyi methods, along with their principles and procedures. It highlights how food processing affects carbohydrate content and bioavailability, detailing changes such as gelatinization, retrogradation, and dietary fiber modification. The document also covers the digestion and absorption of carbohydrates in the human gastrointestinal tract, emphasizing the roles of different monosaccharides and dietary fibers.