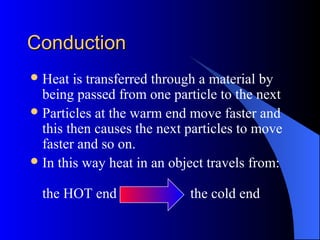
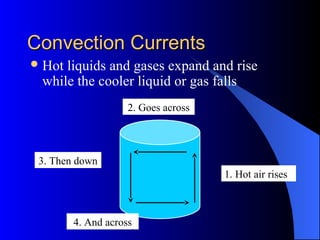
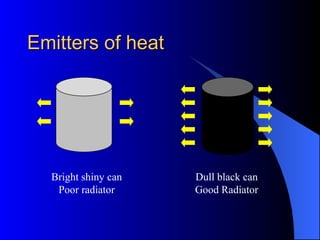
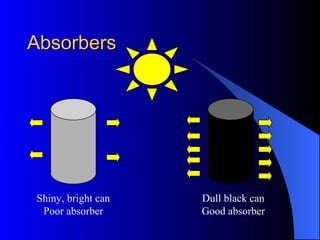
The document explains the concepts of heat energy, temperature, and their interrelationships, including how heat is transferred through conduction, convection, and radiation. It also discusses temperature measurement scales (Fahrenheit, Celsius, Kelvin) and provides examples of heat transfer in various materials. Additionally, it highlights the properties of conductors and insulators in relation to heat transfer.